Abstract
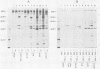
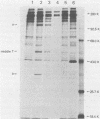
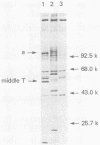
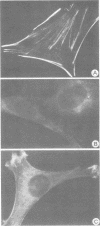
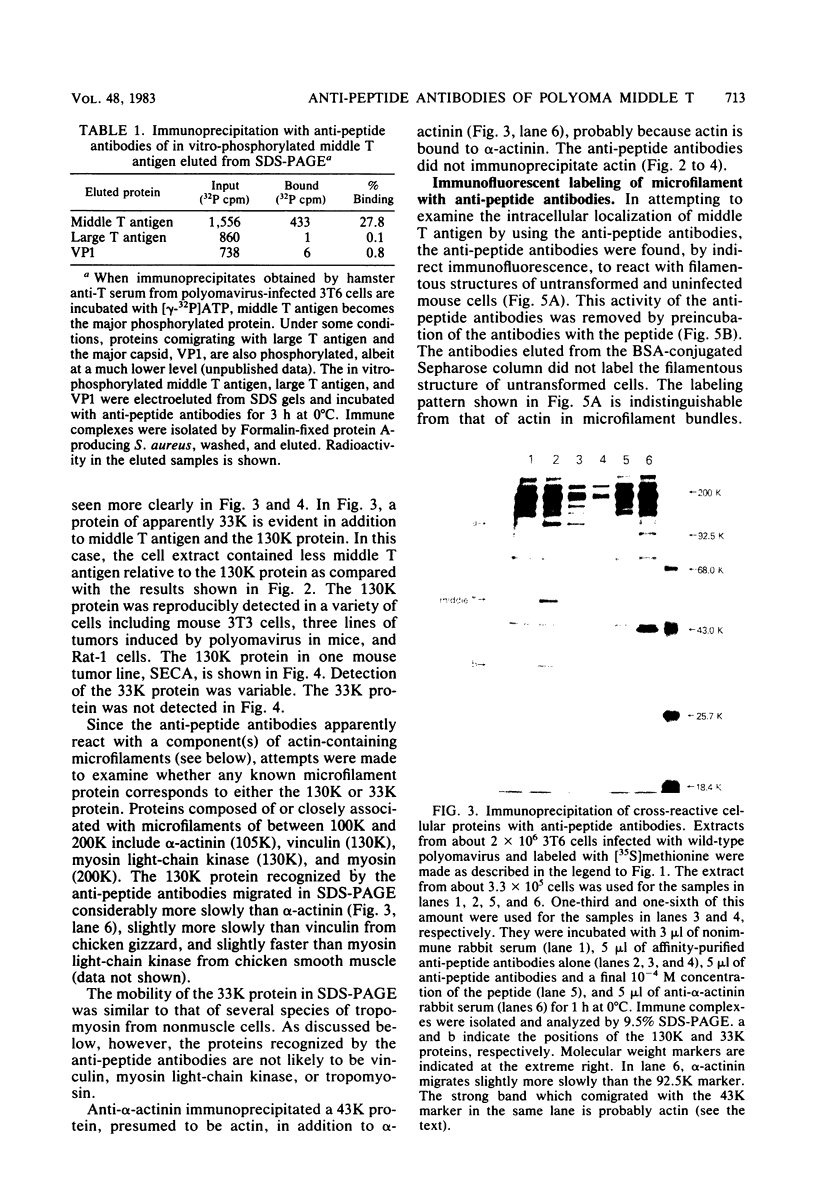
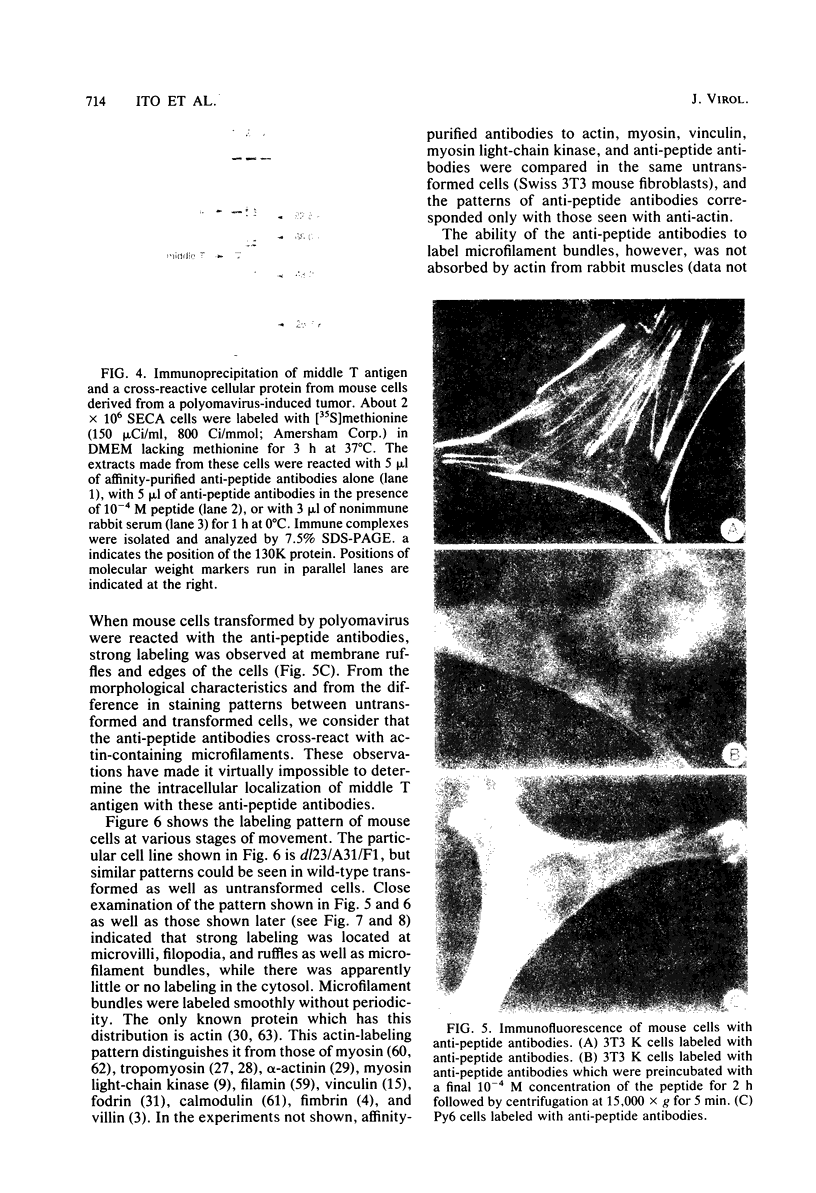
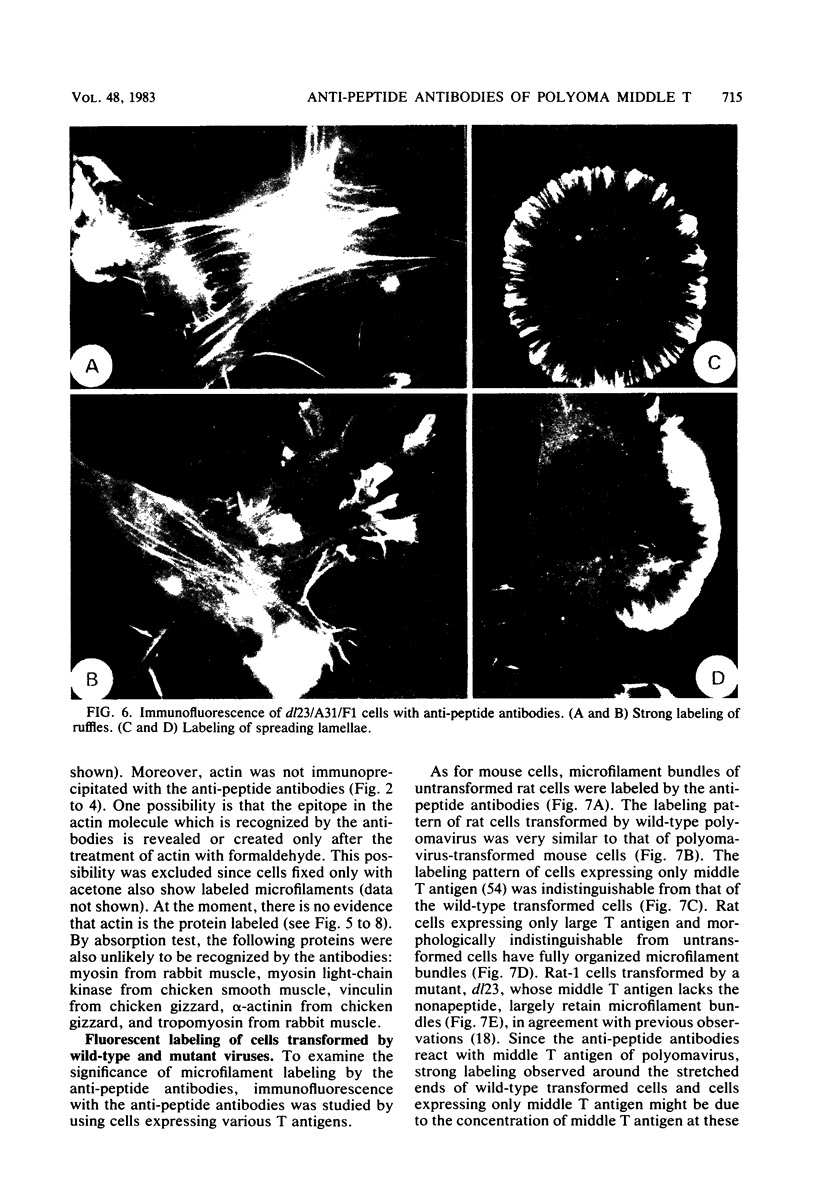
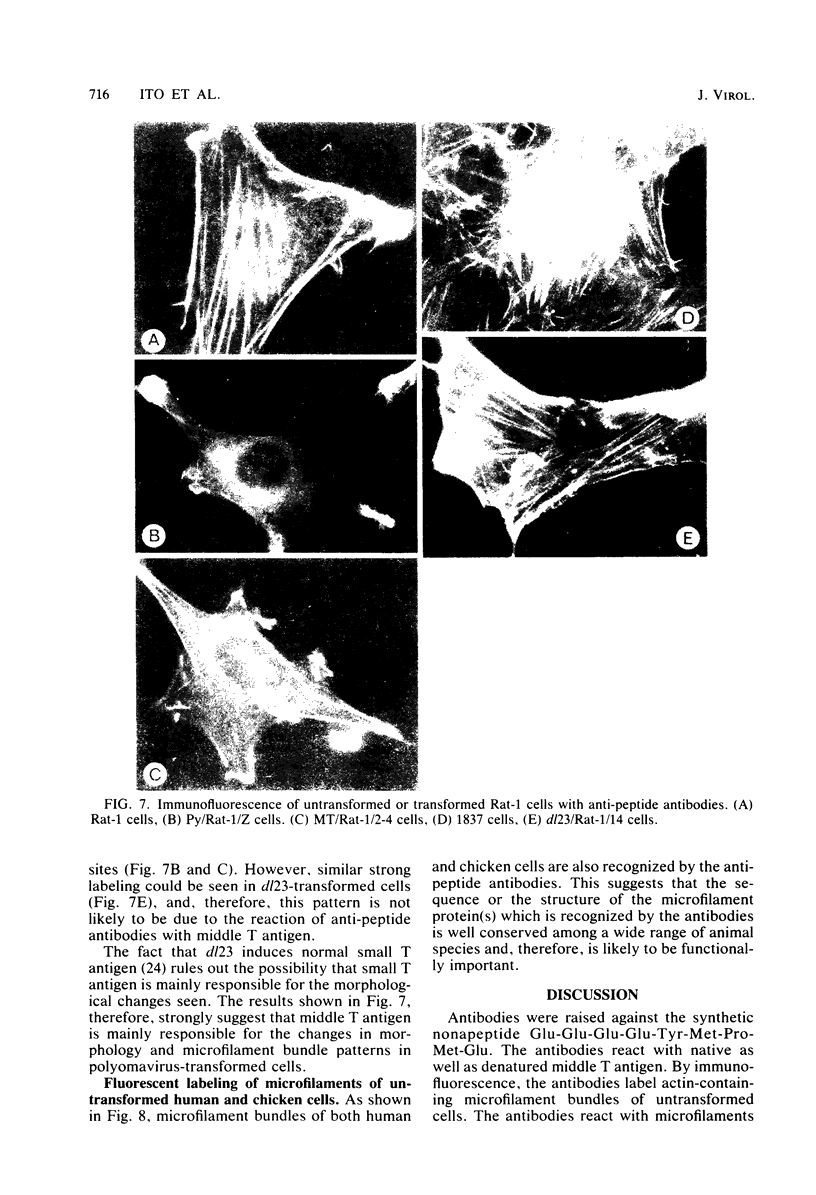
Antibodies were raised against the sequence Glu-Glu-Glu-Glu-Tyr-Met-Pro-Met -Glu, which represents a part of the middle T antigen of polyomavirus that is considered to be important in inducing the phenotype of transformed cells. The antibodies reacted with native as well as denatured middle T antigens. In addition, the antibodies immunoprecipitated a cellular protein with an apparent molecular weight of 130,000 (130K) from mouse and rat cells. In some cases, a 33K protein was also immunoprecipitated. Immunoprecipitation of middle T antigen as well as 130K and 33K proteins was blocked by the peptide. The antibodies labeled microfilaments of untransformed mouse, rat, human, and chicken cells by immunofluorescence. This labeling was also blocked by the peptide. The labeling pattern and distribution under a variety of conditions were indistinguishable from those of anti-actin antibodies, although no evidence has been obtained to indicate that the anti-peptide antibodies react with actin. The 130K protein migrated in sodium dodecyl sulfate-polyacrylamide gel electrophoresis slightly slower than chicken gizzard vinculin (130K) and slightly faster than myosin light-chain kinase of chicken smooth muscle (130K). Neither of these proteins absorbed the anti-peptide antibodies. The 33K protein does not seem to be tropomyosin (32K to 40K).
Full text
PDF


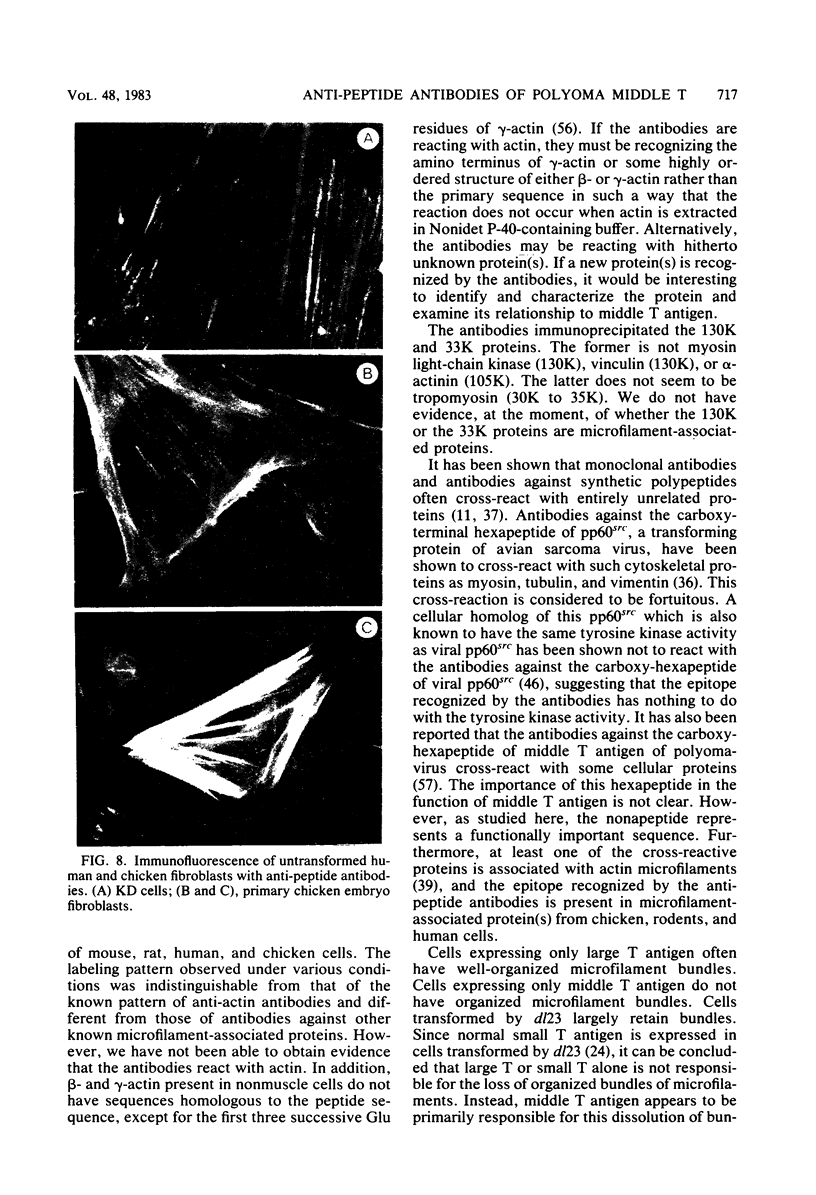



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bendig M. M., Thomas T., Folk W. R. Viable deletion mutant in the medium and large T-antigen-coding sequences of the polyoma virus genome. J Virol. 1980 Mar;33(3):1215–1220. doi: 10.1128/jvi.33.3.1215-1220.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin T. L. Host range mutants of polyoma virus. Proc Natl Acad Sci U S A. 1970 Sep;67(1):394–399. doi: 10.1073/pnas.67.1.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher A., Weber K. Fimbrin, a new microfilament-associated protein present in microvilli and other cell surface structures. J Cell Biol. 1980 Jul;86(1):335–340. doi: 10.1083/jcb.86.1.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher A., Weber K. Villin is a major protein of the microvillus cytoskeleton which binds both G and F actin in a calcium-dependent manner. Cell. 1980 Jul;20(3):839–847. doi: 10.1016/0092-8674(80)90330-x. [DOI] [PubMed] [Google Scholar]
- Chowdhury K., Light S. E., Garon C. F., Ito Y., Israel M. A. A cloned polyoma DNA fragment representing the 5' half of the early gene region is oncogenic. J Virol. 1980 Nov;36(2):566–574. doi: 10.1128/jvi.36.2.566-574.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalianis T., Magnusson G., Ito Y., Klein G. Immunization against the polyoma virus-induced tumor-specific transplantation antigen by early region mutants of the virus. J Virol. 1982 Sep;43(3):772–777. doi: 10.1128/jvi.43.3.772-777.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day R. S., 3rd Studies on repair of adenovirus 2 by human fibroblasts using normal, xeroderma pigmentosum, and xeroderma pigmentosum heterozygous strains. Cancer Res. 1974 Aug;34(8):1965–1970. [PubMed] [Google Scholar]
- Deninger P. L., Esty A., LaPorte P., Hsu H., Friedmann T. The nucleotide sequence and restriction enzyme sites of the polyoma genome. Nucleic Acids Res. 1980 Feb 25;8(4):855–860. [PMC free article] [PubMed] [Google Scholar]
- Ding D., Dilworth S. M., Griffin B. E. mlt Mutants of polyoma virus. J Virol. 1982 Dec;44(3):1080–1083. doi: 10.1128/jvi.44.3.1080-1083.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulbecco R., Unger M., Bologna M., Battifora H., Syka P., Okada S. Cross-reactivity between Thy-1 and a component of intermediate filaments demonstrated using a monoclonal antibody. Nature. 1981 Aug 20;292(5825):772–774. doi: 10.1038/292772a0. [DOI] [PubMed] [Google Scholar]
- Eckhart W., Hutchinson M. A., Hunter T. An activity phosphorylating tyrosine in polyoma T antigen immunoprecipitates. Cell. 1979 Dec;18(4):925–933. doi: 10.1016/0092-8674(79)90205-8. [DOI] [PubMed] [Google Scholar]
- Eckhart W. Polyoma T antigens. Adv Cancer Res. 1981;35:1–25. doi: 10.1016/s0065-230x(08)60906-9. [DOI] [PubMed] [Google Scholar]
- Fried M., Griffin B. E., Lund E., Robberson D. L. Polyoma virus--a study of wild-type, mutant and defective DNAs. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):45–52. doi: 10.1101/sqb.1974.039.01.009. [DOI] [PubMed] [Google Scholar]
- Geiger B. A 130K protein from chicken gizzard: its localization at the termini of microfilament bundles in cultured chicken cells. Cell. 1979 Sep;18(1):193–205. doi: 10.1016/0092-8674(79)90368-4. [DOI] [PubMed] [Google Scholar]
- Griffin B. E., Dilworth S. M. Polyomavirus: an overview of its unique properties. Adv Cancer Res. 1983;39:183–268. doi: 10.1016/s0065-230x(08)61036-2. [DOI] [PubMed] [Google Scholar]
- Griffin B. E., Ito Y., Novak U., Spurr N., Dilworth S., Smolar N., Pollack R., Smith K., Rifkin D. B. Early mutants of polyoma virus (dl8 and dl23) with altered transformation properties: is polyoma virus middle T antigen a transforming gene product? Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):271–283. doi: 10.1101/sqb.1980.044.01.031. [DOI] [PubMed] [Google Scholar]
- Griffin B. E., Maddock C. New classes of viable deletion mutants in the early region of polyoma virus. J Virol. 1979 Sep;31(3):645–656. doi: 10.1128/jvi.31.3.645-656.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori J., Carmichael G. G., Benjamin T. L. DNA sequence alterations in Hr-t deletion mutants of polyoma virus. Cell. 1979 Mar;16(3):505–513. doi: 10.1016/0092-8674(79)90025-4. [DOI] [PubMed] [Google Scholar]
- Hutchinson M. A., Hunter T., Eckhart W. Characterization of T antigens in polyoma-infected and transformed cells. Cell. 1978 Sep;15(1):65–77. doi: 10.1016/0092-8674(78)90083-1. [DOI] [PubMed] [Google Scholar]
- Ito Y., Brocklehurst J. R., Dulbecco R. Virus-specific proteins in the plasma membrane of cells lytically infected or transformed by pol-oma virus. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4666–4670. doi: 10.1073/pnas.74.10.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
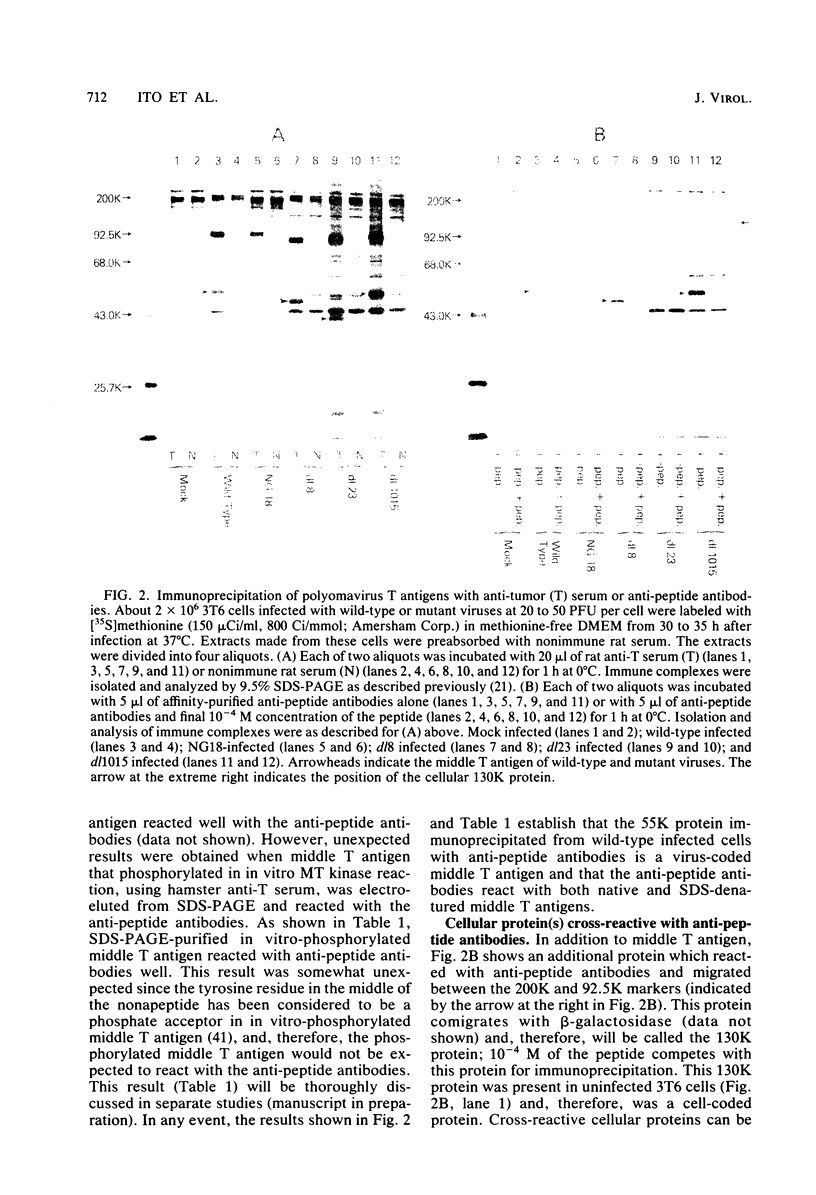
- Ito Y. Polyoma virus-specific 55K protein isolated from plasma membrane of productively infected cells is virus-coded and important for cell transformation. Virology. 1979 Oct 15;98(1):261–266. doi: 10.1016/0042-6822(79)90545-2. [DOI] [PubMed] [Google Scholar]
- Ito Y., Spurr N., Griffin B. E. Middle T antigen as primary inducer of full expression of the phenotype of transformation by polyoma virus. J Virol. 1980 Jul;35(1):219–232. doi: 10.1128/jvi.35.1.219-232.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakunaga T. Neoplastic transformation of human diploid fibroblast cells by chemical carcinogens. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1334–1338. doi: 10.1073/pnas.75.3.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lania L., Griffiths M., Cooke B., Ito Y., Fried M. Untransformed rat cells containing free and integrated DNA of a polyoma nontransforming (Hr-t) mutant. Cell. 1979 Nov;18(3):793–802. doi: 10.1016/0092-8674(79)90132-6. [DOI] [PubMed] [Google Scholar]
- Lazarides E., Burridge K. Alpha-actinin: immunofluorescent localization of a muscle structural protein in nonmuscle cells. Cell. 1975 Nov;6(3):289–298. doi: 10.1016/0092-8674(75)90180-4. [DOI] [PubMed] [Google Scholar]
- Lazarides E. Tropomyosin antibody: the specific localization of tropomyosin in nonmuscle cells. J Cell Biol. 1975 Jun;65(3):549–561. doi: 10.1083/jcb.65.3.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarides E. Two general classes of cytoplasmic actin filaments in tissue culture cells: the role of tropomyosin. J Supramol Struct. 1976;5(4):531(383)–563(415). doi: 10.1002/jss.400050410. [DOI] [PubMed] [Google Scholar]
- Lazarides E., Weber K. Actin antibody: the specific visualization of actin filaments in non-muscle cells. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2268–2272. doi: 10.1073/pnas.71.6.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine J., Willard M. Fodrin: axonally transported polypeptides associated with the internal periphery of many cells. J Cell Biol. 1981 Sep;90(3):631–642. doi: 10.1083/jcb.90.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson G., Berg P. Construction and analysis of viable deletion mutants of polyoma virus. J Virol. 1979 Nov;32(2):523–529. doi: 10.1128/jvi.32.2.523-529.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson G., Nilsson M. G., Dilworth S. M., Smolar N. Characterization of polyoma mutants with altered middle and large T-antigens. J Virol. 1981 Sep;39(3):673–683. doi: 10.1128/jvi.39.3.673-683.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrifield R. B. Solid-phase peptide synthesis. Adv Enzymol Relat Areas Mol Biol. 1969;32:221–296. doi: 10.1002/9780470122778.ch6. [DOI] [PubMed] [Google Scholar]
- Morhenn V., Rabinowitz Z., Tomkins G. M. Effects of adrenal glucocorticoids on polyoma virus replication. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1088–1089. doi: 10.1073/pnas.70.4.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg E. A., Sefton B. M., Hunter T., Walter G., Singer S. J. Immunofluorescent localization of the transforming protein of Rous sarcoma virus with antibodies against a synthetic src peptide. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5322–5326. doi: 10.1073/pnas.79.17.5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg E. A., Walter G., Singer S. J. On the nature of crossreactions observed with antibodies directed to defined epitopes. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5939–5943. doi: 10.1073/pnas.79.19.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson S. V., Tyndall C., Magnusson G. Deletion mapping of a short polyoma virus middle T antigen segment important for transformation. J Virol. 1983 Apr;46(1):284–287. doi: 10.1128/jvi.46.1.284-287.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack R., Osborn M., Weber K. Patterns of organization of actin and myosin in normal and transformed cultured cells. Proc Natl Acad Sci U S A. 1975 Mar;72(3):994–998. doi: 10.1073/pnas.72.3.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassoulzadegan M., Cowie A., Carr A., Glaichenhaus N., Kamen R., Cuzin F. The roles of individual polyoma virus early proteins in oncogenic transformation. Nature. 1982 Dec 23;300(5894):713–718. doi: 10.1038/300713a0. [DOI] [PubMed] [Google Scholar]
- Schaffhausen B. S., Benjamin T. L. Phosphorylation of polyoma T antigens. Cell. 1979 Dec;18(4):935–946. doi: 10.1016/0092-8674(79)90206-x. [DOI] [PubMed] [Google Scholar]
- Schaffhausen B. S., Silver J. E., Benjamin T. L. Tumor antigen(s) in cell productively infected by wild-type polyoma virus and mutant NG-18. Proc Natl Acad Sci U S A. 1978 Jan;75(1):79–83. doi: 10.1073/pnas.75.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffhausen B., Benjamin T. L. Comparison of phosphorylation of two polyoma virus middle T antigens in vivo and in vitro. J Virol. 1981 Oct;40(1):184–196. doi: 10.1128/jvi.40.1.184-196.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffhausen B., Benjamin T. L., Pike L., Casnellie J., Krebs E. Antibody to the nonapeptide Glu-Glu-Glu-Glu-Tyr-Met-Pro-Met-Glu is specific for polyoma middle T antigen and inhibits in vitro kinase activity. J Biol Chem. 1982 Nov 10;257(21):12467–12470. [PubMed] [Google Scholar]
- Schlegel R., Benjamin T. L. Cellular alterations dependent upon the polyoma virus Hr-t function: separation of mitogenic from transforming capacities. Cell. 1978 Jul;14(3):587–599. doi: 10.1016/0092-8674(78)90244-1. [DOI] [PubMed] [Google Scholar]
- Sefton B. M., Walter G. Antiserum specific for the carboxy terminus of the transforming protein of Rous sarcoma virus. J Virol. 1982 Nov;44(2):467–474. doi: 10.1128/jvi.44.2.467-474.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segawa K., Ito Y. Differential subcellular localization of in vivo-phosphorylated and nonphosphorylated middle-sized tumor antigen of polyoma virus and its relationship to middle-sized tumor antigen phosphorylating activity in vitro. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6812–6816. doi: 10.1073/pnas.79.22.6812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart J. E., Ito Y. Three species of polyoma virus tumor antigens share common peptides probably near the amino termini of the proteins. Cell. 1978 Dec;15(4):1427–1437. doi: 10.1016/0092-8674(78)90066-1. [DOI] [PubMed] [Google Scholar]
- Smith A. E., Fried M., Ito Y., Spurr N., Smith R. Is polyoma virus middle T antigen a protein kinase? Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):141–147. doi: 10.1101/sqb.1980.044.01.016. [DOI] [PubMed] [Google Scholar]
- Smith A. E., Smith R., Griffin B., Fried M. Protein kinase activity associated with polyoma virus middle T antigen in vitro. Cell. 1979 Dec;18(4):915–924. doi: 10.1016/0092-8674(79)90204-6. [DOI] [PubMed] [Google Scholar]
- Smolar N., Griffin B. E. DNA sequences of polyoma virus early deletion mutants. J Virol. 1981 Jun;38(3):958–967. doi: 10.1128/jvi.38.3.958-967.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeda E., Arrand J. R., Smolar N., Walsh J. E., Griffin B. E. Coding potential and regulatory signals of the polyoma virus genome. Nature. 1980 Jan 31;283(5746):445–453. doi: 10.1038/283445a0. [DOI] [PubMed] [Google Scholar]
- Soeda E., Griffin B. E. Sequences from the genome of a non-transforming mutant of polyoma virus. Nature. 1978 Nov 16;276(5685):294–298. doi: 10.1038/276294a0. [DOI] [PubMed] [Google Scholar]
- Treisman R., Novak U., Favaloro J., Kamen R. Transformation of rat cells by an altered polyoma virus genome expressing only the middle-T protein. Nature. 1981 Aug 13;292(5824):595–600. doi: 10.1038/292595a0. [DOI] [PubMed] [Google Scholar]
- Türler H. The tumor antigens and the early functions of polyoma virus. Mol Cell Biochem. 1980 Sep 15;32(2):63–93. doi: 10.1007/BF00227801. [DOI] [PubMed] [Google Scholar]
- Vandekerckhove J., Weber K. Actin amino-acid sequences. Comparison of actins from calf thymus, bovine brain, and SV40-transformed mouse 3T3 cells with rabbit skeletal muscle actin. Eur J Biochem. 1978 Oct 16;90(3):451–462. doi: 10.1111/j.1432-1033.1978.tb12624.x. [DOI] [PubMed] [Google Scholar]
- Walter G., Hutchinson M. A., Hunter T., Eckhart W. Antibodies specific for the polyoma virus middle-size tumor antigen. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4882–4886. doi: 10.1073/pnas.78.8.4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter G., Hutchinson M. A., Hunter T., Eckhart W. Purification of polyoma virus medium-size tumor antigen by immunoaffinity chromatography. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4025–4029. doi: 10.1073/pnas.79.13.4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Groeschel-Stewart U. Antibody to myosin: the specific visualization of myosin-containing filaments in nonmuscle cells. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4561–4564. doi: 10.1073/pnas.71.11.4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willingham M. C., Wehland J., Klee C. B., Richert N. D., Rutherford A. V., Pastan I. H. Ultrastructural immunocytochemical localization of calmodulin in cultured cells. J Histochem Cytochem. 1983 Apr;31(4):445–461. doi: 10.1177/31.4.6338107. [DOI] [PubMed] [Google Scholar]
- Willingham M. C., Yamada S. S., Bechtel P. J., Rutherford A. V., Pastan I. H. Ultrastructural immunocytochemical localization of myosin in cultured fibroblastic cells. J Histochem Cytochem. 1981 Nov;29(11):1289–1301. doi: 10.1177/29.11.7033361. [DOI] [PubMed] [Google Scholar]
- Willingham M. C., Yamada S. S., Davies P. J., Rutherford A. V., Gallo M. G., Pastan I. Intracellular localization of actin in cultured fibroblasts by electron microscopic immunocytochemistry. J Histochem Cytochem. 1981 Jan;29(1):17–37. doi: 10.1177/29.1.7009728. [DOI] [PubMed] [Google Scholar]
- de Lanerolle P., Adelstein R. S., Feramisco J. R., Burridge K. Characterization of antibodies to smooth muscle myosin kinase and their use in localizing myosin kinase in nonmuscle cells. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4738–4742. doi: 10.1073/pnas.78.8.4738. [DOI] [PMC free article] [PubMed] [Google Scholar]