Abstract
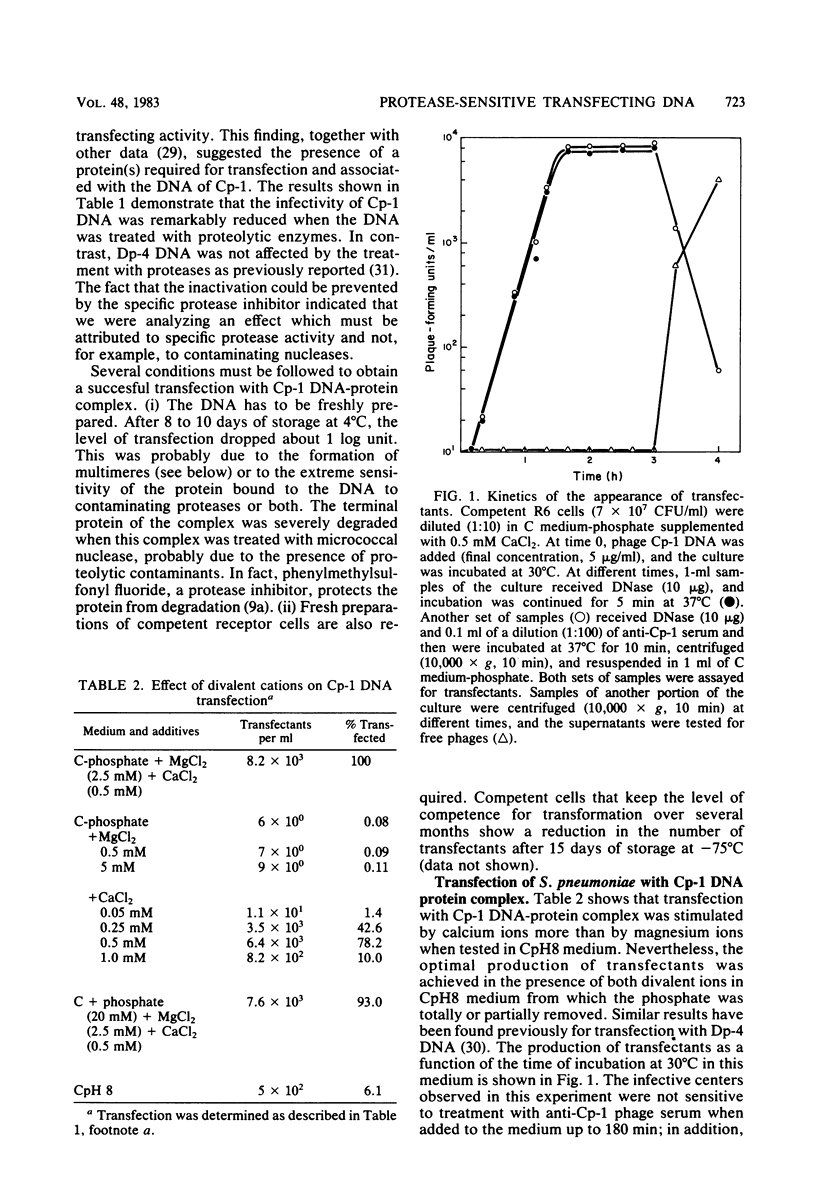
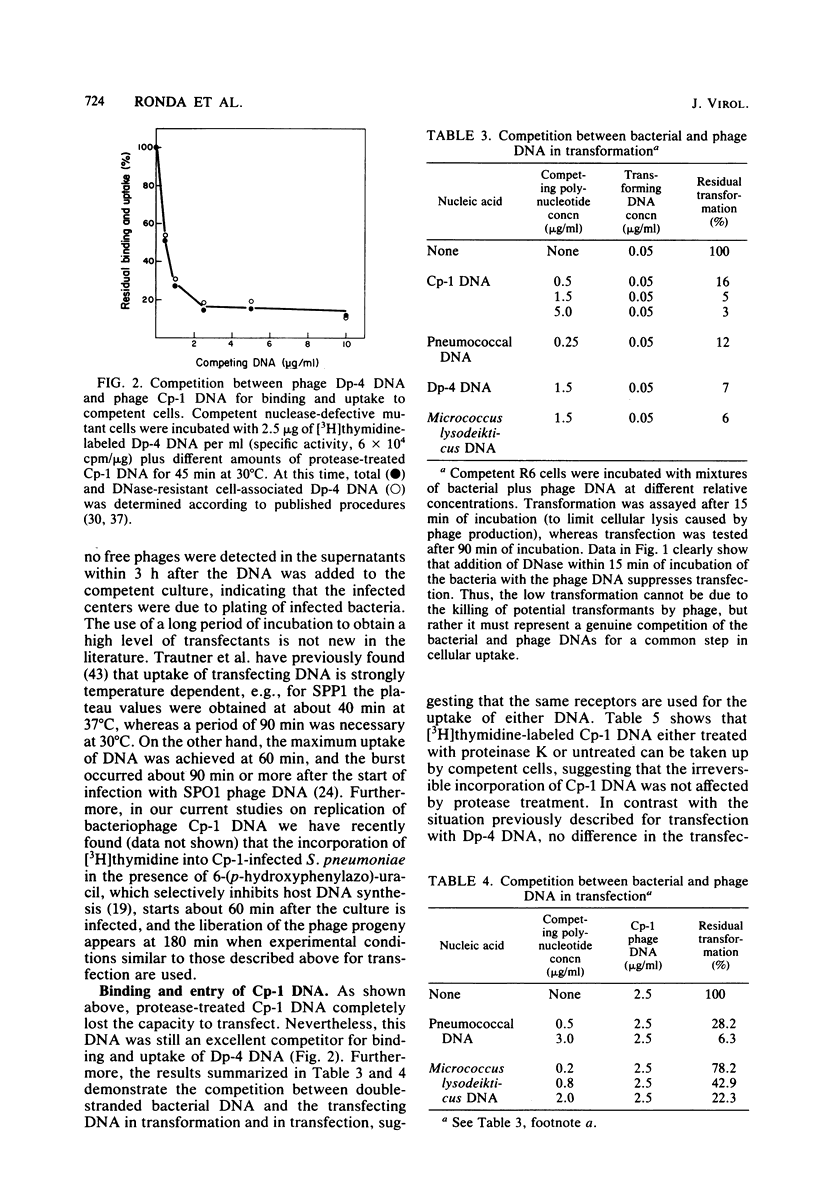
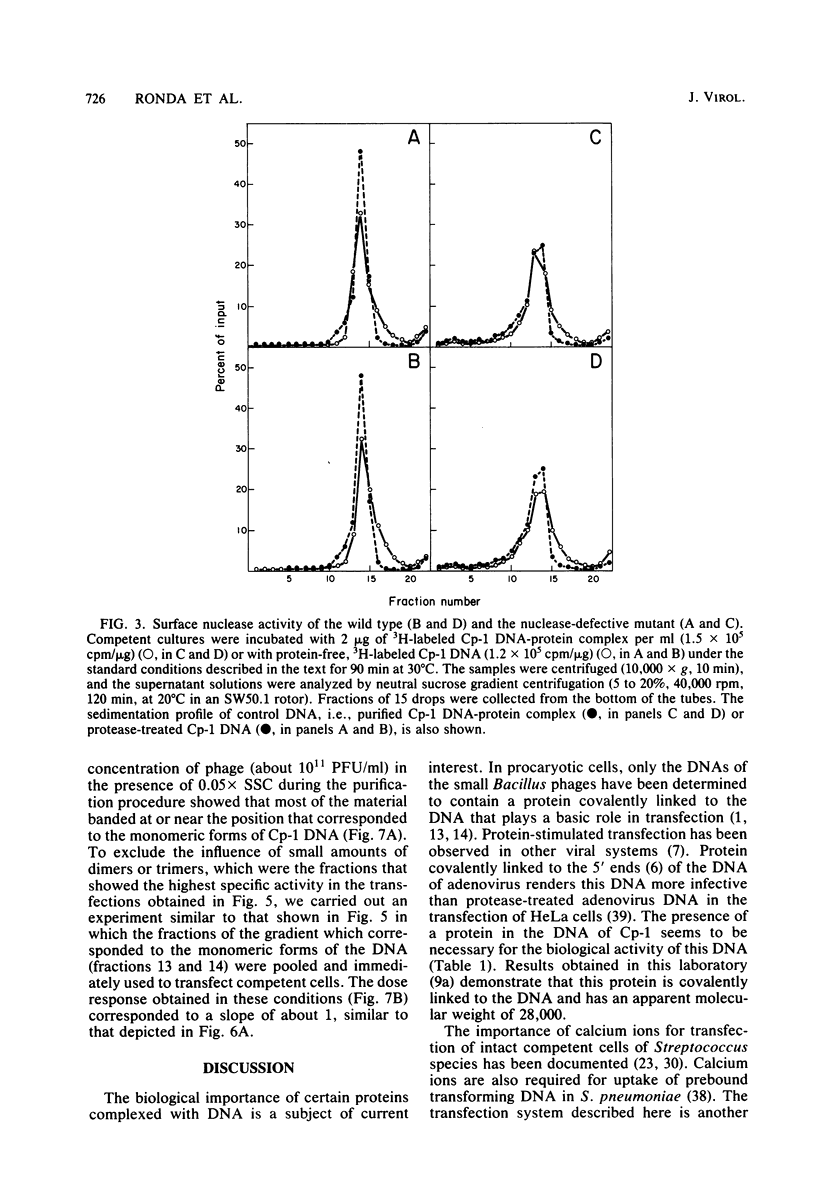
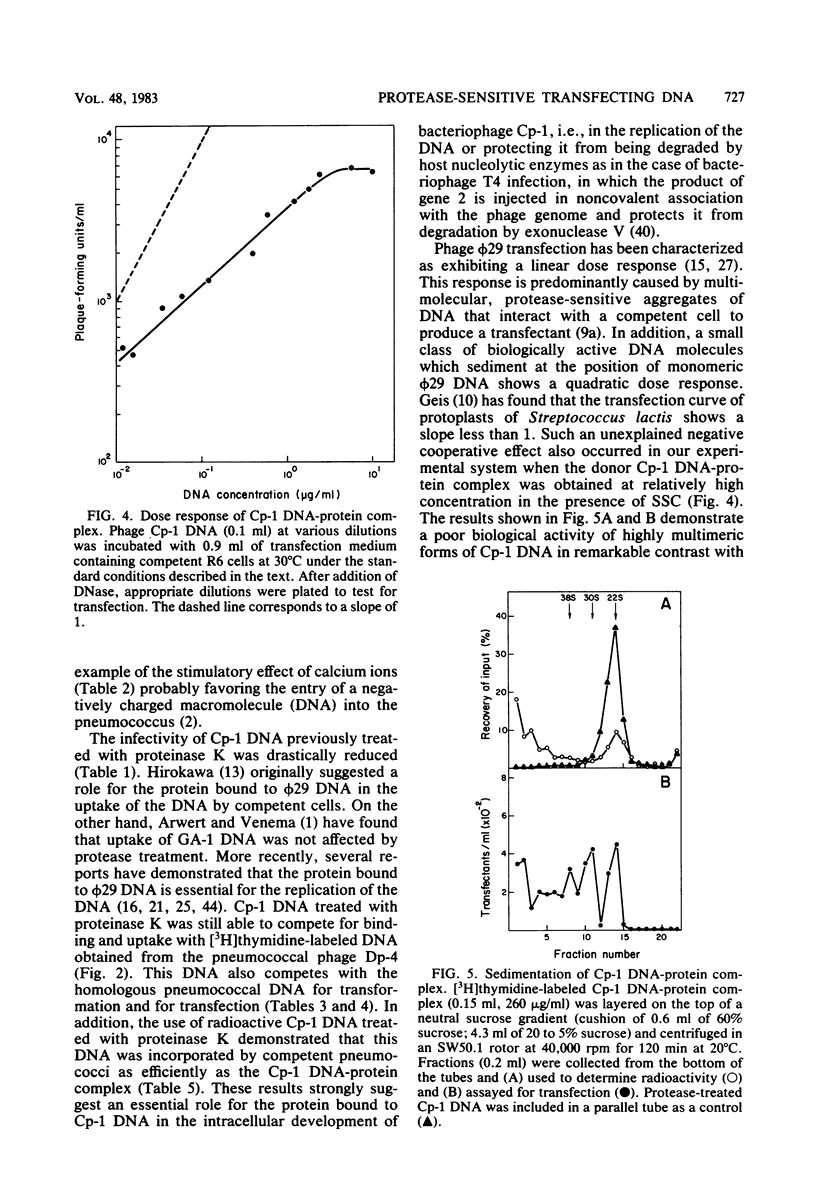
The transfecting activity of pneumococcal phage Cp-1 DNA was destroyed by treatment with proteolytic enzymes, although these enzymes did not affect transfection with bacteriophage Dp-4 DNA. This transfection was stimulated by calcium ions. Protease-treated Cp-1 DNA competes for binding and uptake with transforming pneumococcal DNA as well as with transfecting Dp-4 DNA to approximately the same extent as does untreated Cp-1 DNA. In addition, [3H]thymidine-labeled Cp-1 DNA, treated with proteases or untreated, was absorbed with the same efficiency. These data suggest that uptake of Cp-1 DNA is not affected by protease treatment. [3H]thymidine-labeled Cp-1 DNA showed remarkable resistance against surface nuclease activity of competent wild-type cells. The monomeric form of the Cp-1 DNA-protein complex showed a linear dose response in transfection.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arwert F., Venema G. Protease-sensitive transfection of Bacillus subtilis with bacteriophage GA-1 DNA: a probable case of heterologous transfection. J Virol. 1974 Mar;13(3):584–589. doi: 10.1128/jvi.13.3.584-589.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barany F., Tomasz A. Genetic transformation of Streptococcus pneumoniae by heterologous plasmid deoxyribonucleic acid. J Bacteriol. 1980 Nov;144(2):698–709. doi: 10.1128/jb.144.2.698-709.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnke D. Plasmid transformation of Streptococcus sanguis (Challis) occurs by circular and linear molecules. Mol Gen Genet. 1981;182(3):490–497. doi: 10.1007/BF00293940. [DOI] [PubMed] [Google Scholar]
- Canosi U., Morelli G., Trautner T. A. The relationship between molecular structure and transformation efficiency of some S. aureus plasmids isolated from B. subtilis. Mol Gen Genet. 1978 Nov 9;166(3):259–267. doi: 10.1007/BF00267617. [DOI] [PubMed] [Google Scholar]
- Carusi E. A. Evidence for blocked 5'-termini in human adenovirus DNA. Virology. 1977 Jan;76(1):380–394. doi: 10.1016/0042-6822(77)90310-5. [DOI] [PubMed] [Google Scholar]
- Chase C. D., Benzinger R. H. Transfection of Escherichia coli spheroplasts with a bacteriophage Mu DNA-protein complex. J Virol. 1982 Apr;42(1):176–185. doi: 10.1128/jvi.42.1.176-185.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García E., Gómez A., Ronda C., Escarmis C., López R. Pneumococcal bacteriophage Cp-1 contains a protein bound to the 5' termini of its DNA. Virology. 1983 Jul 15;128(1):92–104. doi: 10.1016/0042-6822(83)90321-5. [DOI] [PubMed] [Google Scholar]
- Goscin L. P., Guild W. R. Enhancement of pneumococcal transfection by protamine sulfate. J Bacteriol. 1982 Nov;152(2):765–772. doi: 10.1128/jb.152.2.765-772.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermoso J. M., Salas M. Protein p3 is linked to the DNA of phage phi 29 through a phosphoester bond between serine and 5'-dAMP. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6425–6428. doi: 10.1073/pnas.77.11.6425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa H. Transfecting deoxyribonucleic acid of Bacillus bacteriophage phi 29 that is protease sensitive. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1555–1559. doi: 10.1073/pnas.69.6.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa H., Trautner T. A., Lüder G. On the dose response in B. subtilis transfection: involvement of aggregates in phi 29 transfection. Mol Gen Genet. 1977 Nov 18;156(3):263–266. doi: 10.1007/BF00267180. [DOI] [PubMed] [Google Scholar]
- Ito J. Bacteriophage phi29 terminal protein: its association with the 5' termini of the phi29 genome. J Virol. 1978 Dec;28(3):895–904. doi: 10.1128/jvi.28.3.895-904.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacks S., Greenberg B. Single-strand breakage on binding of DNA to cells in the genetic transformation of Diplococcus pneumoniae. J Mol Biol. 1976 Feb 25;101(2):255–275. doi: 10.1016/0022-2836(76)90376-4. [DOI] [PubMed] [Google Scholar]
- Lacks S. Uptake of circular deoxyribonucleic acid and mechanism of deoxyribonucleic acid transport in genetic transformation of Streptococcus pneumoniae. J Bacteriol. 1979 May;138(2):404–409. doi: 10.1128/jb.138.2.404-409.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez R., Ronda C., Tomasz A., Portoles A. Properties of "diplophage": a lipid-containing bacteriophage. J Virol. 1977 Oct;24(1):201–210. doi: 10.1128/jvi.24.1.201-210.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López R., García E., Ronda C. Selective replication of diplophage Dp-4 deoxyribonucleic acid in 6-(p-hydroxyphenylazo)-uracil treated Streptococcus pneumoniae. FEBS Lett. 1980 Feb 25;111(1):66–68. doi: 10.1016/0014-5793(80)80762-9. [DOI] [PubMed] [Google Scholar]
- Mellado R. P., Peñalva M. A., Inciarte M. R., Salas M. The protein covalently linked to the 5' termini of the DNA of Bacillus subtilis phage phi 29 is involved in the initiation of DNA replication. Virology. 1980 Jul 15;104(1):84–96. doi: 10.1016/0042-6822(80)90367-0. [DOI] [PubMed] [Google Scholar]
- Michel B., Niaudet B., Ehrlich S. D. Intramolecular recombination during plasmid transformation of Bacillus subtilis competent cells. EMBO J. 1982;1(12):1565–1571. doi: 10.1002/j.1460-2075.1982.tb01356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent K. M., Cole R. M. Characterization of group H streptococcal temperate bacteriophage phi 227. J Virol. 1977 Mar;21(3):1061–1073. doi: 10.1128/jvi.21.3.1061-1073.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OKUBO S., STRAUSS B., STODOLSKY M. THE POSSIBLE ROLE OF RECOMBINATION IN THE INFECTION OF COMPETENT BACILLUS SUBTILIS BY BACTERIOPHAGE DEOXYRIBONUCLEIC ACID. Virology. 1964 Dec;24:552–562. doi: 10.1016/0042-6822(64)90207-7. [DOI] [PubMed] [Google Scholar]
- Peñalva M. A., Salas M. Initiation of phage phi 29 DNA replication in vitro: formation of a covalent complex between the terminal protein, p3, and 5'-dAMP. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5522–5526. doi: 10.1073/pnas.79.18.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter R. D., Guild W. R. Transfection in pneumococcus: single-strand intermediates in the formation of infective centers. J Virol. 1978 Jan;25(1):60–72. doi: 10.1128/jvi.25.1.60-72.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REILLY B. E., SPIZIZEN J. BACTERIOPHAGE DEOXYRIBONUCLEATE INFECTION OF COMPETENT BACILLUS SUBTILIS. J Bacteriol. 1965 Mar;89:782–790. doi: 10.1128/jb.89.3.782-790.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roger M. Evidence for conversion of heteroduplex transforming DNAs to homoduplexes by recipient pneumococcal cells (DNA strand resolution-DNA repair-bacterial transformation-genetic recombination). Proc Natl Acad Sci U S A. 1972 Feb;69(2):466–470. doi: 10.1073/pnas.69.2.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronda C., Lopez R., Tomasz A., Portoles A. Transfection of Streptococcus pneumoniae with bacteriophage DNA. J Virol. 1978 May;26(2):221–225. doi: 10.1128/jvi.26.2.221-225.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronda C., López R., García E. Isolation and characterization of a new bacteriophage, Cp-1, infecting Streptococcus pneumoniae. J Virol. 1981 Nov;40(2):551–559. doi: 10.1128/jvi.40.2.551-559.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg A. H., Simon M. N., Studier F. W., Roberts R. J. Survey and mapping of restriction endonuclease cleavage sites in bacteriophage T7 DNA. J Mol Biol. 1979 Dec 25;135(4):907–915. doi: 10.1016/0022-2836(79)90519-9. [DOI] [PubMed] [Google Scholar]
- Saunders C. W., Guild W. R. Pathway of plasmid transformation in Pneumococcus: open circular and linear molecules are active. J Bacteriol. 1981 May;146(2):517–526. doi: 10.1128/jb.146.2.517-526.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto H., Lopez R., Garrigan O., Tomasz A. Nucleolytic degradation of homologous and heterologous deoxyribonucleic acid molecules at the surface of competent pneumococci. J Bacteriol. 1975 May;122(2):676–685. doi: 10.1128/jb.122.2.676-685.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto H., Lopez R., Tomasz A. Cell surface-located deoxyribonucleic acid receptors in transformable pneumococci. J Bacteriol. 1975 Jun;122(3):1339–1350. doi: 10.1128/jb.122.3.1339-1350.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto H., Tomasz A. Calcium-requiring step in the uptake of deoxyribonucleic acid molecules through the surface of competent pneumococci. J Bacteriol. 1976 Jun;126(3):1113–1118. doi: 10.1128/jb.126.3.1113-1118.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto H., Tomasz A. Early stages in DNA binding and uptake during genetic transformation of pneumococci. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1493–1498. doi: 10.1073/pnas.71.4.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A., Moore C., Haverty J. L. The infectivity of adenovirus 5 DNA-protein complex. Virology. 1976 Dec;75(2):442–456. doi: 10.1016/0042-6822(76)90042-8. [DOI] [PubMed] [Google Scholar]
- Silverstein J. L., Goldberg E. B. T4 DNA injection. II. Protection of entering DNA from host exonuclease V. Virology. 1976 Jul 1;72(1):212–223. doi: 10.1016/0042-6822(76)90324-x. [DOI] [PubMed] [Google Scholar]
- Tomasz A. Cellular metabolism in genetic transformation of pneumococci: requirement for protein synthesis during induction of competence. J Bacteriol. 1970 Mar;101(3):860–871. doi: 10.1128/jb.101.3.860-871.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A. Model for the mechanism controlling the expression of competent state in Pneumococcus cultures. J Bacteriol. 1966 Mar;91(3):1050–1061. doi: 10.1128/jb.91.3.1050-1061.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautner T. A., Spatz H. C. Transfection in B. subtilis. Curr Top Microbiol Immunol. 1973;62:61–88. doi: 10.1007/978-3-642-65772-6_3. [DOI] [PubMed] [Google Scholar]
- Watabe K., Shih M. F., Sugino A., Ito J. In vitro replication of bacteriophage phi 29 DNA. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5245–5248. doi: 10.1073/pnas.79.17.5245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanofsky S., Kawamura F., Ito J. Thermolabile transfecting DNA from temperature-sensitive mutant of phage phi29. Nature. 1976 Jan 1;259(5538):60–63. doi: 10.1038/259060a0. [DOI] [PubMed] [Google Scholar]


