Abstract
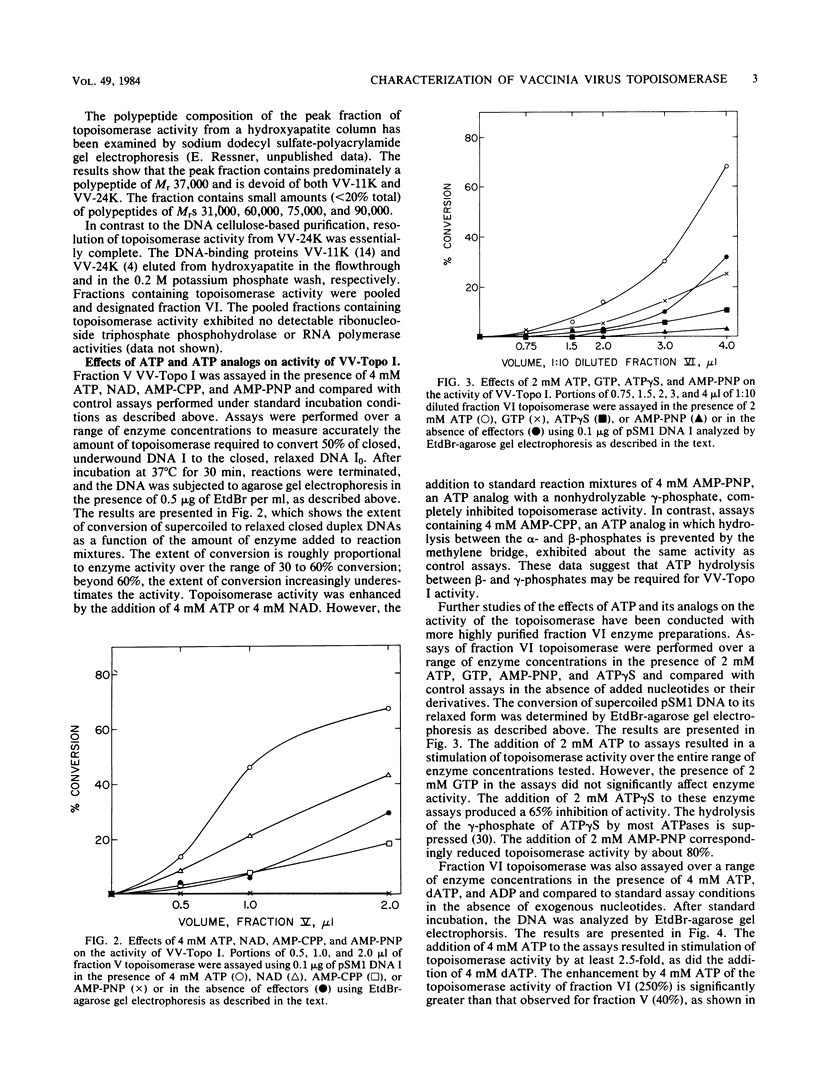
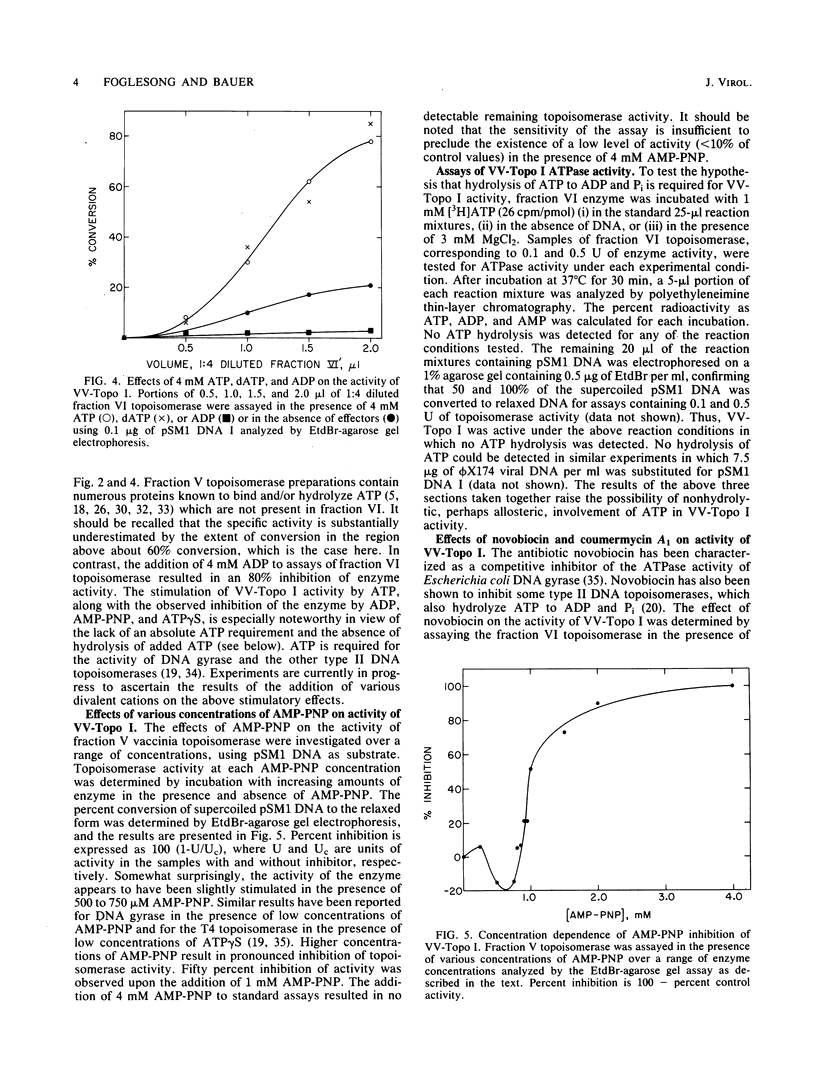
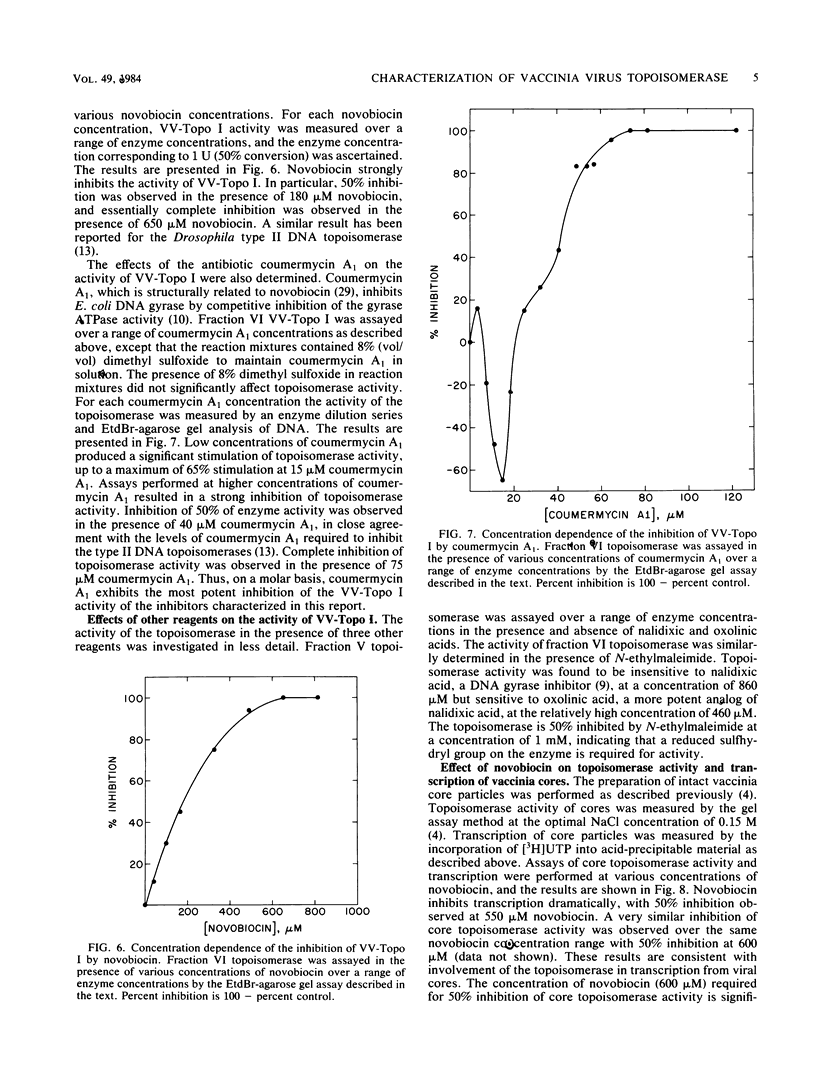
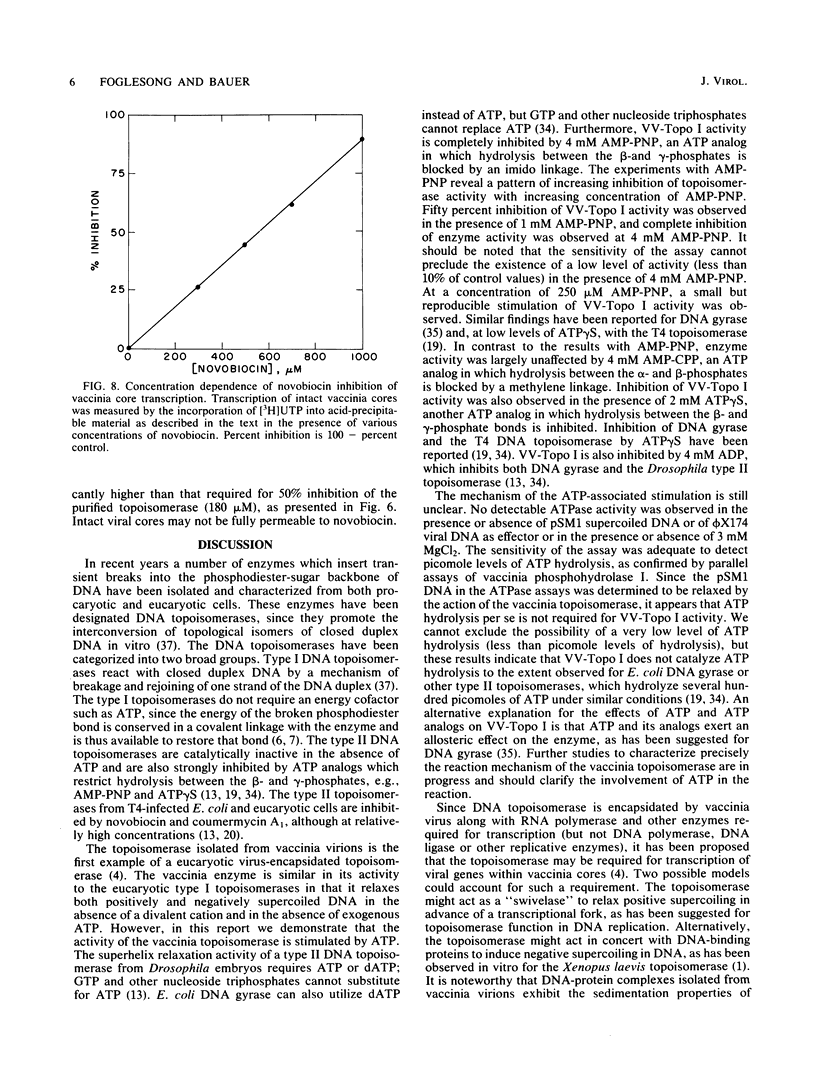
Vaccinia virus cores contain a type I topoisomerase which promotes the relaxation of superhelical DNA of either handedness (Bauer et al., Proc. Natl. Acad. Sci. U.S.A. 74:1841-1845, 1977). The activity of partially purified vaccinia virus topoisomerase (VV-Topo I) was determined in the presence of ATP, dATP, GTP, ADP, and ATP analogs in which hydrolysis of the alpha, beta or beta, gamma phosphate bond is restricted. Topoisomerase activity was stimulated 2.5-fold by the addition of 2 to 4 mM ATP or dATP to standard assay mixtures; 2 mM GTP produced no significant effect on enzyme activity. The addition of 2 mM beta, gamma-imido ATP or 2 mM gamma-thiophosphate ATP reduced VV-Topo I activity by 80 and 65%, respectively. In contrast, 4 mM alpha, beta-methylene ATP produced no significant change in topoisomerase activity compared to ATP itself. Assays performed in the presence of 4 mM ADP exhibited an 80% reduction in enzyme activity. The preparations of VV-Topo I used for these studies showed, however, no detectable DNA-dependent or -independent ATPase activity. The activity of VV-Topo I was similarly measured in the presence of the antibiotics novobiocin and coumermycin A1, which inhibited enzyme activity by 50% at concentrations of 180 and 40 microM, respectively. Comparable inhibition of VV-Topo I activity was observed in the presence of 1 mM beta, gamma-imido ATP. We determined that novobiocin inhibits vaccinia core transcription at the same concentrations which inhibit vaccinia core topoisomerase I activity. These results suggest that the vaccinia DNA topoisomerase may play a role in the ATP-dependent transcription of viral genes from intact core particles.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldi M. I., Mattoccia E., Tocchini-Valentini G. P. DNA supercoiling by Xenopus laevis oocyte extracts: requirement for a nuclear factor. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4873–4876. doi: 10.1073/pnas.75.10.4873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa E., Moss B. mRNA(nucleoside-2'-)-methyltransferase from vaccinia virus. Purification and physical properties. J Biol Chem. 1978 Nov 10;253(21):7692–7697. [PubMed] [Google Scholar]
- Baroudy B. M., Moss B. Purification and characterization of a DNA-dependent RNA polymerase from vaccinia virions. J Biol Chem. 1980 May 10;255(9):4372–4380. [PubMed] [Google Scholar]
- Bauer W. R., Ressner E. C., Kates J., Patzke J. V. A DNA nicking-closing enzyme encapsidated in vaccinia virus: partial purification and properties. Proc Natl Acad Sci U S A. 1977 May;74(5):1841–1845. doi: 10.1073/pnas.74.5.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brakel C., Kates J. R. Poly(A) polymerase from vaccinia virus-infected cells. I. Partial purification and characterization. J Virol. 1974 Oct;14(4):715–723. doi: 10.1128/jvi.14.4.715-723.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champoux J. J. Strand breakage by the DNA untwisting enzyme results in covalent attachment of the enzyme to DNA. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3800–3804. doi: 10.1073/pnas.74.9.3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depew R. E., Liu L. F., Wang J. C. Interaction between DNA and Escherichia coli protein omega. Formation of a complex between single-stranded DNA and omega protein. J Biol Chem. 1978 Jan 25;253(2):511–518. [PubMed] [Google Scholar]
- Esteban M., Soloski M., Cabrera C. V., Holowczak J. A. Replication of vaccinia DNA and studies on the structure of the viral chromosome. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):789–799. doi: 10.1101/sqb.1979.043.01.086. [DOI] [PubMed] [Google Scholar]
- Gellert M., Mizuuchi K., O'Dea M. H., Itoh T., Tomizawa J. I. Nalidixic acid resistance: a second genetic character involved in DNA gyrase activity. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4772–4776. doi: 10.1073/pnas.74.11.4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert M., O'Dea M. H., Itoh T., Tomizawa J. Novobiocin and coumermycin inhibit DNA supercoiling catalyzed by DNA gyrase. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4474–4478. doi: 10.1073/pnas.73.12.4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershowitz A., Boone R. F., Moss B. Multiple roles for ATP in the synthesis and processing of mRNA by vaccinia virus: specific inhibitory effects of adenosine (beta,gamma-imido) triphosphate. J Virol. 1978 Aug;27(2):399–408. doi: 10.1128/jvi.27.2.399-408.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruby D. E., Guarino L. A., Kates J. R. Vaccinia virus replication. I. Requirement for the host-cell nucleus. J Virol. 1979 Feb;29(2):705–715. doi: 10.1128/jvi.29.2.705-715.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh T., Brutlag D. ATP-dependent DNA topoisonmerase from D. melanogaster reversibly catenates duplex DNA rings. Cell. 1980 Aug;21(1):115–125. doi: 10.1016/0092-8674(80)90119-1. [DOI] [PubMed] [Google Scholar]
- Kao S. Y., Ressner E., Kates J., Bauer W. R. Purification and characterization of a superhelix binding protein from vaccinia virus. Virology. 1981 Jun;111(2):500–508. doi: 10.1016/0042-6822(81)90352-4. [DOI] [PubMed] [Google Scholar]
- Kates J. R., McAuslan B. R. Messenger RNA synthesis by a "coated" viral genome. Proc Natl Acad Sci U S A. 1967 Feb;57(2):314–320. doi: 10.1073/pnas.57.2.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kates J. R., McAuslan B. R. Poxvirus DNA-dependent RNA polymerase. Proc Natl Acad Sci U S A. 1967 Jul;58(1):134–141. doi: 10.1073/pnas.58.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kates J., Beeson J. Ribonucleic acid synthesis in vaccinia virus. II. Synthesis of polyriboadenylic acid. J Mol Biol. 1970 May 28;50(1):19–33. doi: 10.1016/0022-2836(70)90101-4. [DOI] [PubMed] [Google Scholar]
- Kleiman J. H., Moss B. Purification of a protein kinase and two phosphate acceptor proteins from vaccinia virions. J Biol Chem. 1975 Apr 10;250(7):2420–2429. [PubMed] [Google Scholar]
- Liu L. F., Liu C. C., Alberts B. M. T4 DNA topoisomerase: a new ATP-dependent enzyme essential for initiation of T4 bacteriophage DNA replication. Nature. 1979 Oct 11;281(5731):456–461. doi: 10.1038/281456a0. [DOI] [PubMed] [Google Scholar]
- Liu L. F., Liu C. C., Alberts B. M. Type II DNA topoisomerases: enzymes that can unknot a topologically knotted DNA molecule via a reversible double-strand break. Cell. 1980 Mar;19(3):697–707. doi: 10.1016/s0092-8674(80)80046-8. [DOI] [PubMed] [Google Scholar]
- Martin S. A., Paoletti E., Moss B. Purification of mRNA guanylyltransferase and mRNA (guanine-7-) methyltransferase from vaccinia virions. J Biol Chem. 1975 Dec 25;250(24):9322–9329. [PubMed] [Google Scholar]
- Mickel S., Bauer W. Isolation, by tetracycline selection, of small plasmids derived from R-factor R12 in Escherichia coli K-12. J Bacteriol. 1976 Jul;127(1):644–655. doi: 10.1128/jb.127.1.644-655.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroy G., Spencer E., Hurwitz J. Purification of mRNA guanylyltransferase from vaccinia virions. J Biol Chem. 1978 Jun 25;253(12):4481–4489. [PubMed] [Google Scholar]
- Moss B., Rosenblum E. N., Gershowitz A. Characterization of a polyriboadenylate polymerase from vaccinia virions. J Biol Chem. 1975 Jun 25;250(12):4722–4729. [PubMed] [Google Scholar]
- Munyon W., Paoletti E., Grace J. T., Jr RNA polymerase activity in purified infectious vaccinia virus. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2280–2287. doi: 10.1073/pnas.58.6.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolette E., Rosemond-Hornbeak H., Moss B. Two nucleid acid-dependent nucleoside triphosphate phosphohydrolases from vaccinia virus. Purification and characterization. J Biol Chem. 1974 May 25;249(10):3273–3280. [PubMed] [Google Scholar]
- Pogo B. G., O'shea M. T. Further characterization of deoxyribonucleases from vaccinia virus. Virology. 1977 Mar;77(1):56–66. doi: 10.1016/0042-6822(77)90405-6. [DOI] [PubMed] [Google Scholar]
- Rosemond-Hornbeak H., Paoletti E., Moss B. Single-stranded deoxyribonucleic acid-specific nuclease from vaccinia virus. Purification and characterization. J Biol Chem. 1974 May 25;249(10):3287–3291. [PubMed] [Google Scholar]
- Ryan M. J. Coumermycin A1: A preferential inhibitor of replicative DNA synthesis in Escherichia coli. I. In vivo characterization. Biochemistry. 1976 Aug 24;15(17):3769–3777. doi: 10.1021/bi00662a020. [DOI] [PubMed] [Google Scholar]
- Shuman S., Spencer E., Furneaux H., Hurwitz J. The role of ATP in in vitro vaccinia virus RNA synthesis effects of AMP-PNP and ATP gamma S. J Biol Chem. 1980 Jun 10;255(11):5396–5403. [PubMed] [Google Scholar]
- Soloski M. J., Holowczak J. A. Characterization of supercoiled nucleoprotein complexes released from detergent-treated vaccinia virions. J Virol. 1981 Feb;37(2):770–783. doi: 10.1128/jvi.37.2.770-783.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer E., Loring D., Hurwitz J., Monroy G. Enzymatic conversion of 5'-phosphate-terminated RNA to 5'-di- and triphosphate-terminated RNA. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4793–4797. doi: 10.1073/pnas.75.10.4793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer E., Shuman S., Hurwitz J. Purification and properties of vaccinia virus DNA-dependent RNA polymerase. J Biol Chem. 1980 Jun 10;255(11):5388–5395. [PubMed] [Google Scholar]
- Sugino A., Cozzarelli N. R. The intrinsic ATPase of DNA gyrase. J Biol Chem. 1980 Jul 10;255(13):6299–6306. [PubMed] [Google Scholar]
- Sugino A., Higgins N. P., Brown P. O., Peebles C. L., Cozzarelli N. R. Energy coupling in DNA gyrase and the mechanism of action of novobiocin. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4838–4842. doi: 10.1073/pnas.75.10.4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosberg H. P., Grossman L. I., Vinograd J. Isolation and partial characterisation of the relaxation protein from nuclei of cultured mouse and human cells. Eur J Biochem. 1975 Jun 16;55(1):79–93. doi: 10.1111/j.1432-1033.1975.tb02140.x. [DOI] [PubMed] [Google Scholar]


