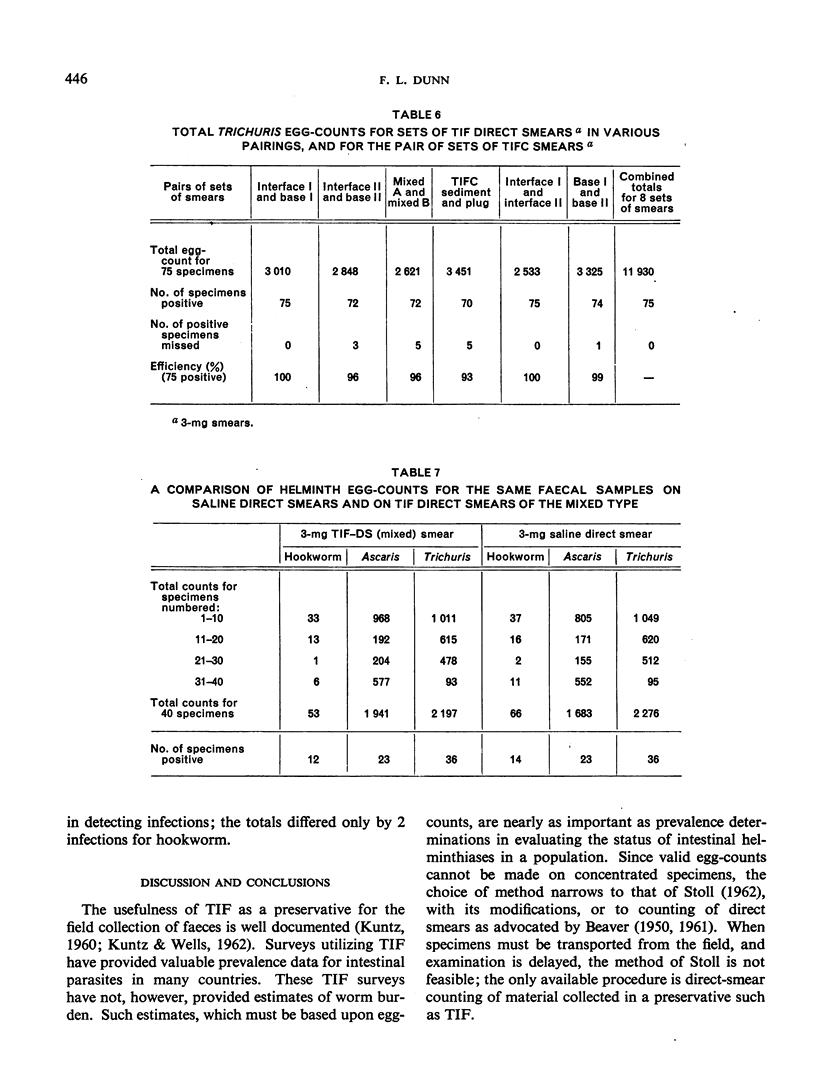
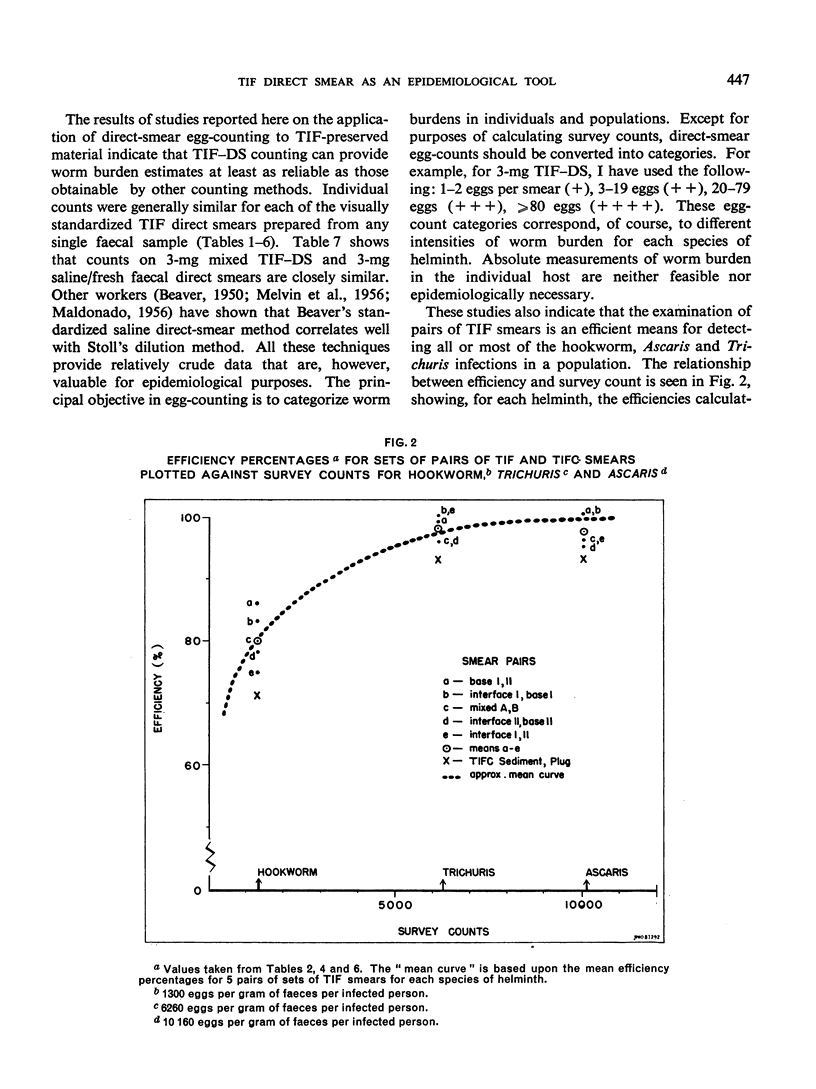
Abstract
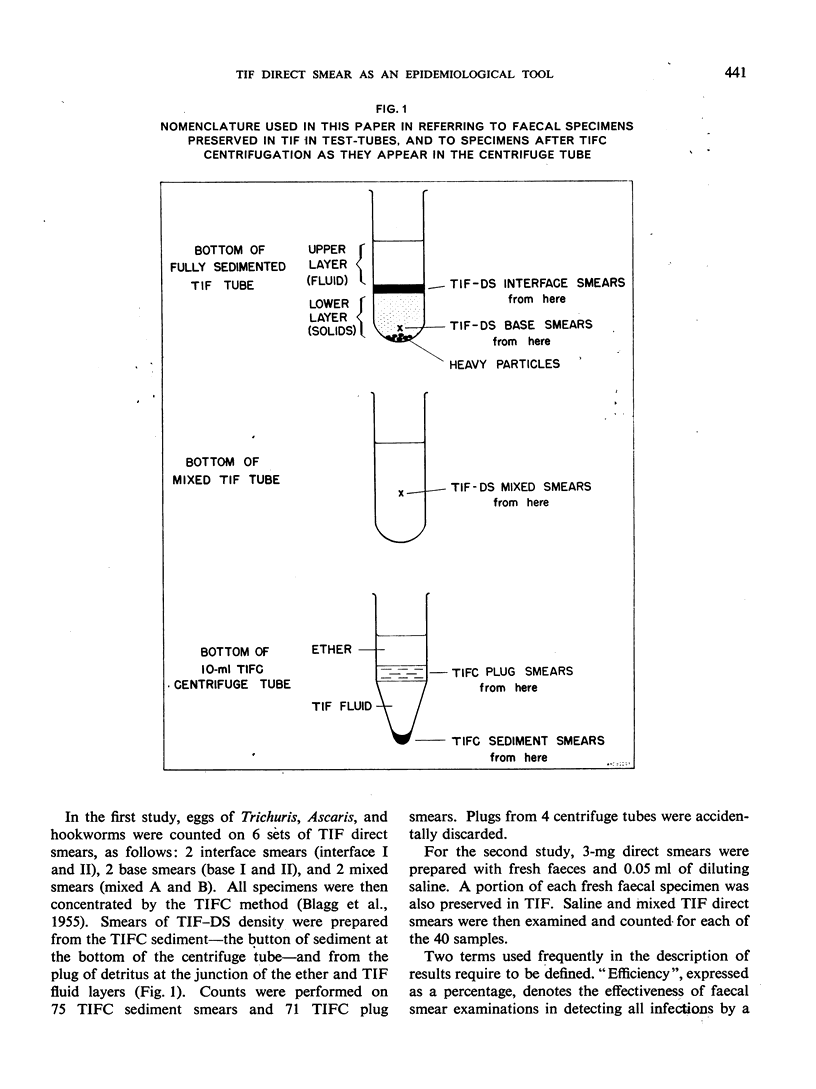
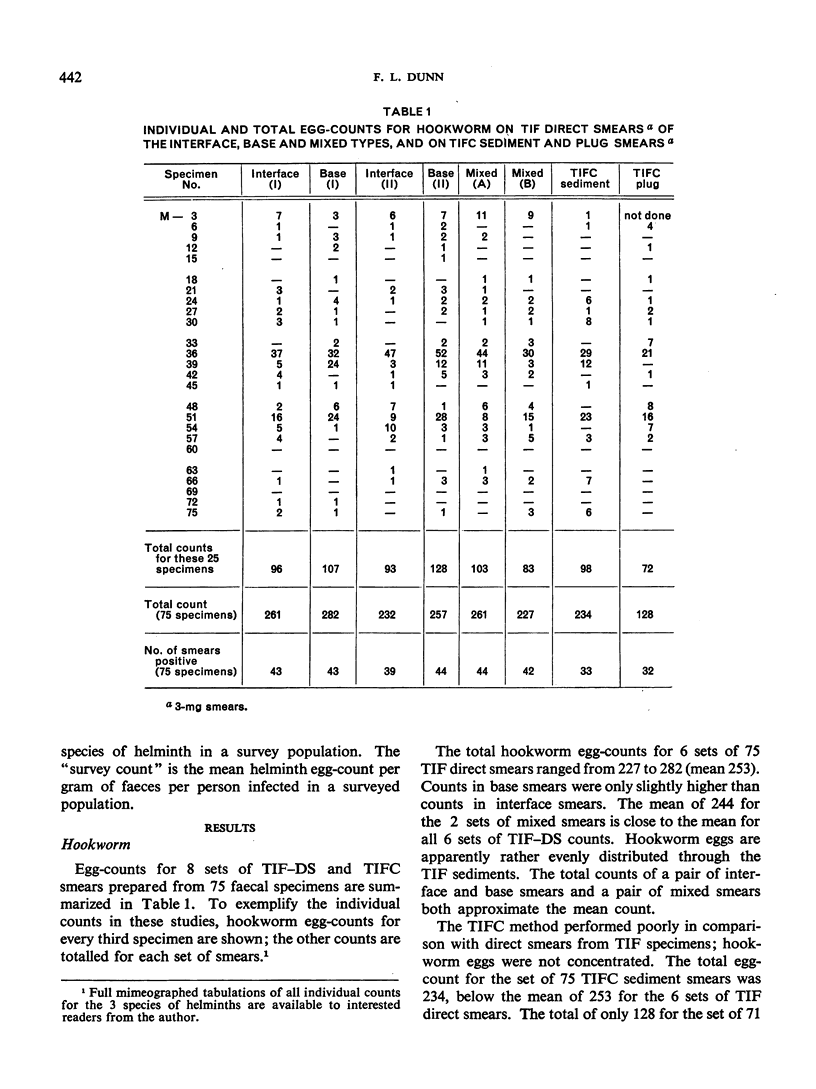
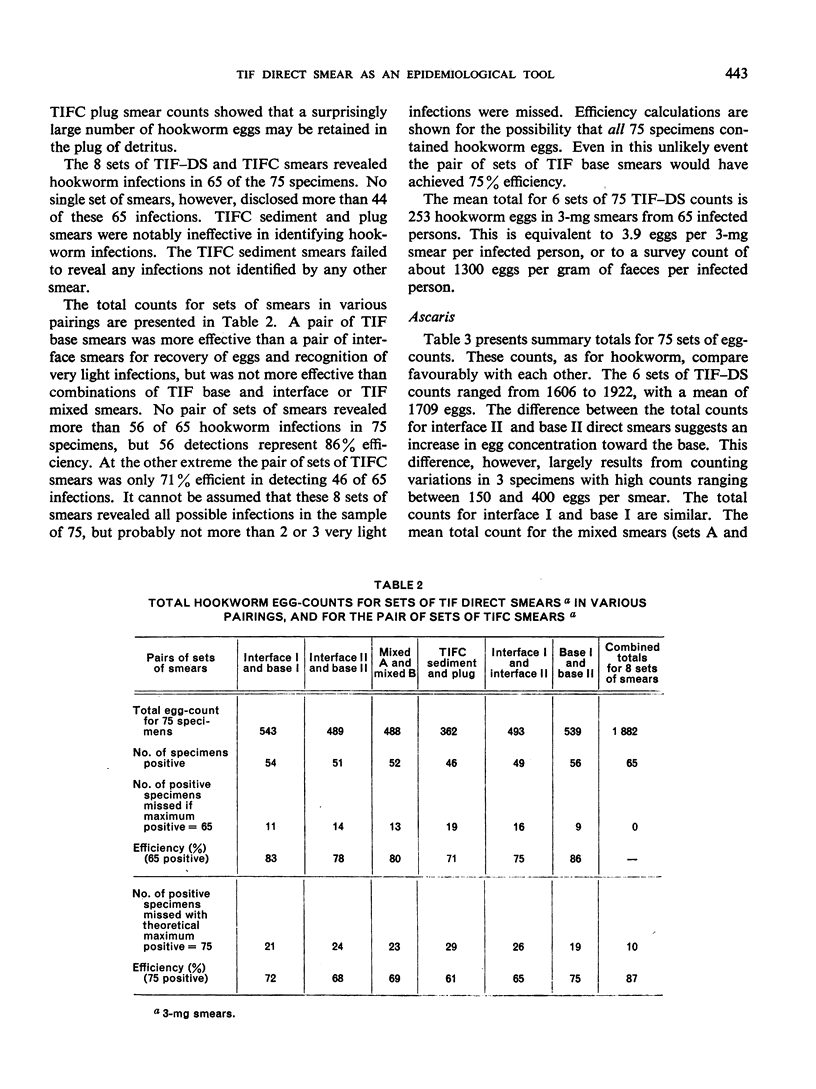
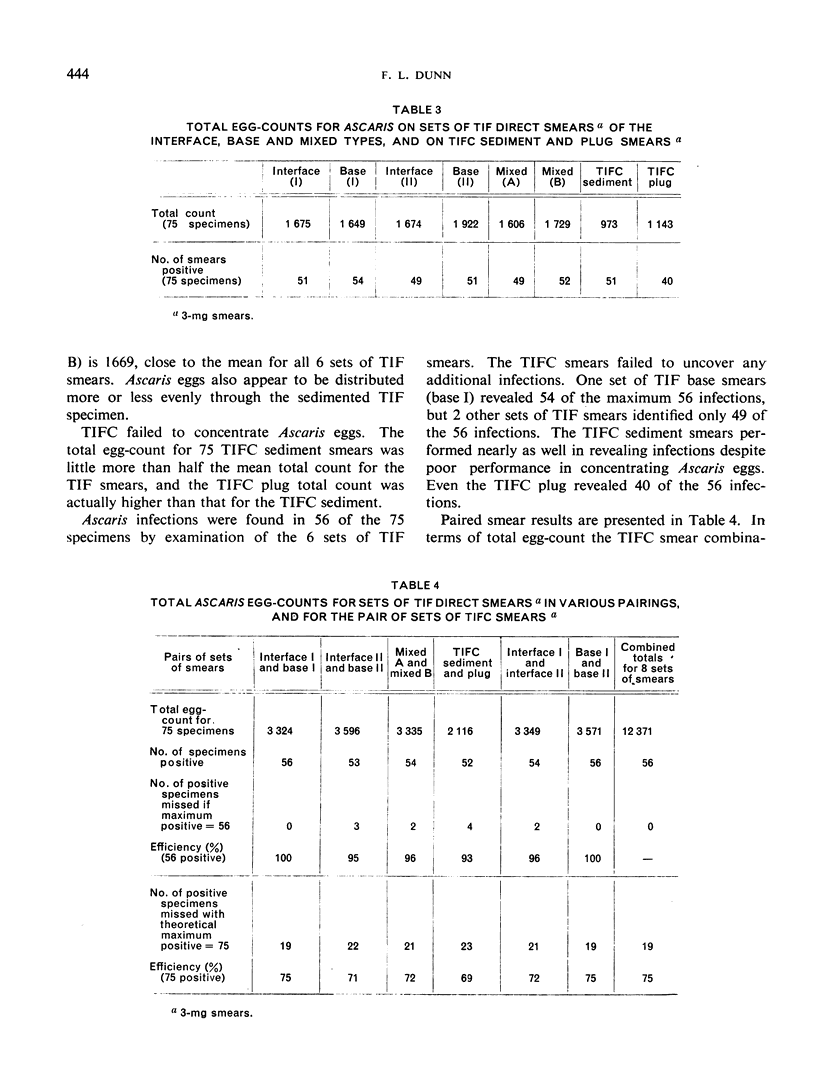
Thiomersal—iodine—formalin (TIF) has been used as a faecal preservative in many prevalence surveys of intestinal helminths and protozoa. In helminth surveys, however, estimates of worm burden are no less essential than those of prevalence. Direct-smear and dilution egg-counting techniques, using fresh faeces, have been developed to provide such estimates. This study originated in a field survey that required the use of a preservative. Attempts to estimate worm burden with TIF-preserved faeces led to the assessment of TIF direct-smear (TIF—DS) methods reported here. TIF—DS egg-counting provides reliable statistical estimates of hookworm, Ascaris and Trichuris burden; this is a satisfactory method for estimating worm burden when faeces must be preserved and transported from the field. TIF direct-smears also determine parasite prevalence “efficiently”. Some previous studies have cast doubt on the value of TIF concentration (TIFC) and in this study also TIFC proved to be erratic or ineffective in concentrating eggs.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEAVER P. C. The standardization of fecal smears for estimating egg production and worm burden. J Parasitol. 1950 Oct;36(5):451–456. [PubMed] [Google Scholar]
- BLAGG W., SCHLOEGEL E. L., MANSOUR N. S., KHALAF G. I. A new concentration technic for the demonstration of protozoa and helminth eggs in feces. Am J Trop Med Hyg. 1955 Jan;4(1):23–28. doi: 10.4269/ajtmh.1955.4.23. [DOI] [PubMed] [Google Scholar]
- HEIMLICH C. R., MELVIN D. M., SADUN E. H. Comparison of the direct smear and dilution egg counts in the quantitative determination of hookworm infections. Am J Hyg. 1956 Sep;64(2):139–148. doi: 10.1093/oxfordjournals.aje.a119829. [DOI] [PubMed] [Google Scholar]
- KUNTZ R. E. Intestinal protozoa and helminths in school children of Dacca, East Pakistan (East Bengal). Am J Trop Med Hyg. 1960 Mar;9:168–172. doi: 10.4269/ajtmh.1960.9.168. [DOI] [PubMed] [Google Scholar]
- KUNTZ R. E., WELLS W. H. Intestinal parasites of man in British North Borneo. Am J Trop Med Hyg. 1962 Nov;11:773–780. doi: 10.4269/ajtmh.1962.11.773. [DOI] [PubMed] [Google Scholar]
- Komiya Y., Kobayashi A. Evaluation of Kato's thick smear technic with a cellophane cover for helminth eggs in feces. Jpn J Med Sci Biol. 1966 Feb;19(1):59–64. doi: 10.7883/yoken1952.19.59. [DOI] [PubMed] [Google Scholar]
- MALDONADO J. F. An evaluation of the standardized direct smear for egg counting in parasitological work. Am J Trop Med Hyg. 1956 Sep;5(5):888–892. doi: 10.4269/ajtmh.1956.5.888. [DOI] [PubMed] [Google Scholar]
- SAPERO J. J., LAWLESS D. K. The MIF stain-preservation technic for the identification of intestinal protozoa. Am J Trop Med Hyg. 1953 Jul;2(4):613–619. doi: 10.4269/ajtmh.1953.2.613. [DOI] [PubMed] [Google Scholar]


