Abstract
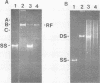
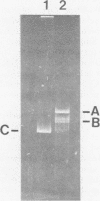
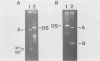
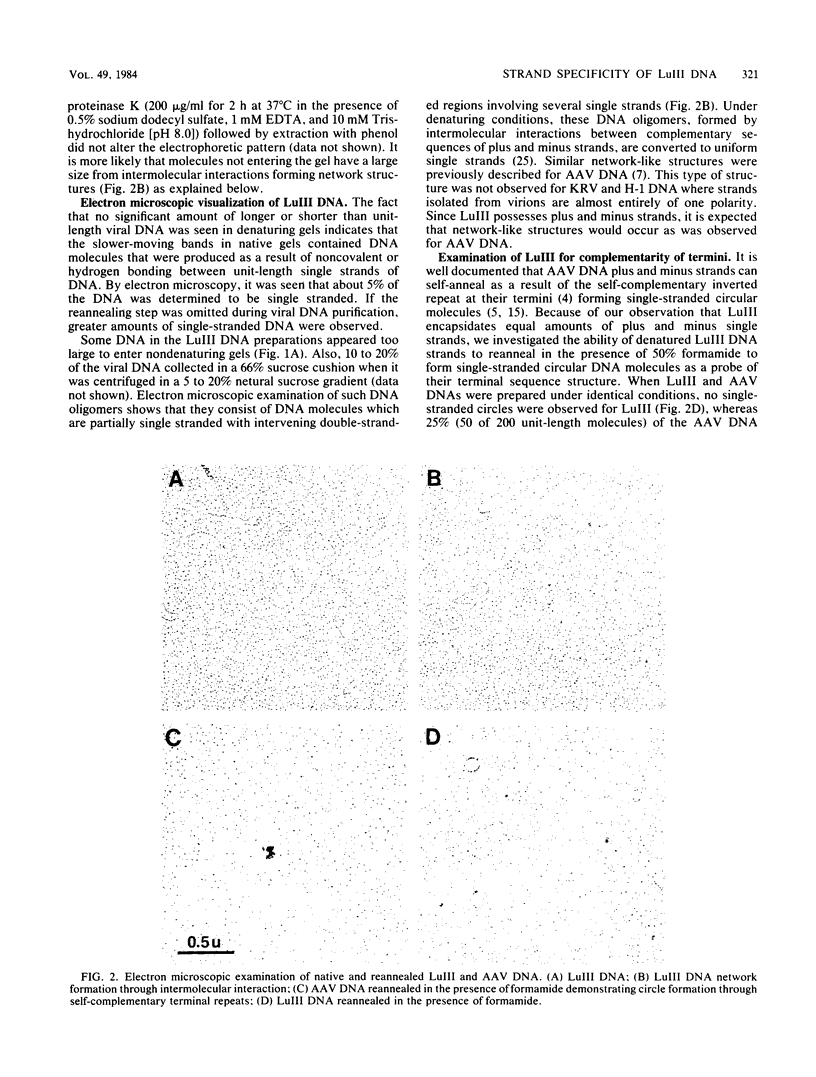
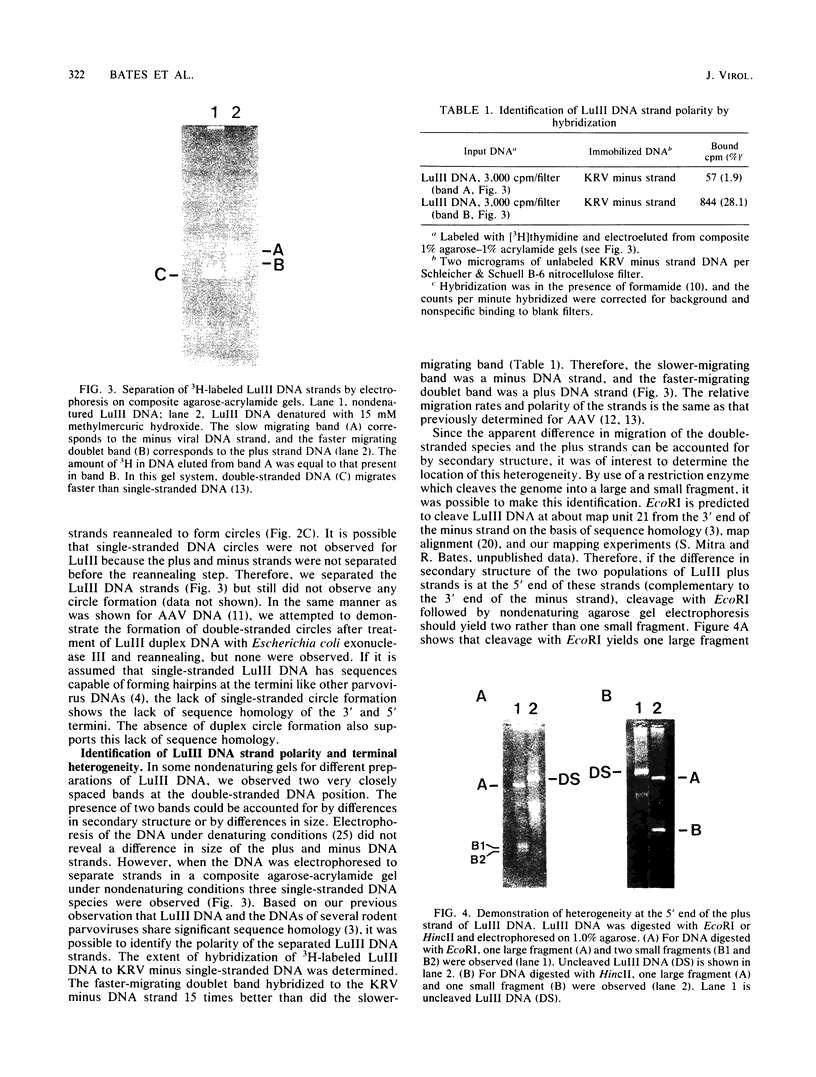
Autonomous parvoviruses are thought to uniquely encapsidate single-stranded DNA of minus polarity. In contrast, the defective adeno-associated viruses separately encapsidate equal amounts of plus and minus DNA strands. We reexamined the uniqueness of minus strand encapsidation for the autonomous parvoviruses. Although we found that Kilham rat virus and H-1 virus encapsidate varying but small amounts of complementary-strand DNA, it was unexpected to find that LuIII virus encapsidated equal amounts of plus and minus DNA. The extracted LuIII DNA possessed properties of double-stranded replicative-form DNA, including insensitivity to S1 endonuclease, cleavage by restriction enzymes, and conversion to unit-length, single-stranded DNA when electrophoresed under denaturing conditions. However, the inability of this DNA to form single-stranded DNA circles when denatured and then renatured in the presence of formamide and the lack of double-stranded DNA circle formation after treatment with exonuclease III and reannealing shows a lack of sequence homology of the 3' and 5' termini of LuIII DNA, in contrast to adeno-associated virus DNA. Digestion of LuIII double-stranded DNA with EcoRI and HincII and separation of plus and minus DNA strands on composite agarose-acrylamide gels identified a heterogeneity present only in the plus DNA strand. These results suggest that strand specificity of viral DNA encapsidation is not a useful property for differentiation between the autonomous and defective parvoviruses. Furthermore, encapsidation by LuIII of equal amounts of complementary DNA strands in contrast to encapsidation of minus strands by H-1 virus, when propagated in the same host cell type, suggests that selection of strands for encapsidation is a virus-coded rather than host-controlled event.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison D. P., Ganesan A. T., Olson A. C., Snyder C. M., Mitra S. Electron microscopic studies of bacteriophage M13 DNA replication. J Virol. 1977 Nov;24(2):673–684. doi: 10.1128/jvi.24.2.673-684.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann P. A., Hoggan M. D., Kurstak E., Melnick J. L., Pereira H. G., Tattersall P., Vago C. Parvoviridae: second report. Intervirology. 1979;11(4):248–254. doi: 10.1159/000149041. [DOI] [PubMed] [Google Scholar]
- Banerjee P. T., Olson W. H., Allison D. P., Bates R. C., Snyder C. E., Mitra S. Electron microscopic comparison of the sequences of single-stranded genomes of mammalian parvoviruses by heteroduplex mapping. J Mol Biol. 1983 May 25;166(3):257–272. doi: 10.1016/s0022-2836(83)80084-9. [DOI] [PubMed] [Google Scholar]
- Berns K. I., Kelly T. J., Jr Letter: Visualization of the inverted terminal repetition in adeno-associated virus DNA. J Mol Biol. 1974 Jan 15;82(2):267–271. doi: 10.1016/0022-2836(74)90344-1. [DOI] [PubMed] [Google Scholar]
- Bourguignon G. J., Tattersall P. J., Ward D. C. DNA of minute virus of mice: self-priming, nonpermuted, single-stranded genome with a 5'-terminal hairpin duplex. J Virol. 1976 Oct;20(1):290–306. doi: 10.1128/jvi.20.1.290-306.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter B. J., Khoury G. Specific cleavage of adenovirus-associated virus DNA by restriction endonuclease R-EcoRI--characterization of cleavage products. Virology. 1975 Feb;63(2):523–538. doi: 10.1016/0042-6822(75)90325-6. [DOI] [PubMed] [Google Scholar]
- Dasgupta S., Allison D. P., Snyder C. E., Mitra S. Base-unpaired regions in supercoiled replicative form DNA of coliphage M13. J Biol Chem. 1977 Aug 25;252(16):5916–5923. [PubMed] [Google Scholar]
- Fujimura R. K. Biochemical analysis of the naturally repaired sections of bacteriophage T5 deoxyribonucleic acid. II. Conditions for nucleotide incorporation under nonpermissive conditions. Biochemistry. 1971 Nov 23;10(24):4381–4386. doi: 10.1021/bi00800a005. [DOI] [PubMed] [Google Scholar]
- Gerry H. W., Kelly T. J., Jr, Berns K. I. Arrangement of nucleotide sequences in adeno-associated virus DNA. J Mol Biol. 1973 Sep 15;79(2):207–225. doi: 10.1016/0022-2836(73)90001-6. [DOI] [PubMed] [Google Scholar]
- Hauswirth W. W., Berns K. I. Origin and termination of adeno-associated virus DNA replication. Virology. 1977 May 15;78(2):488–499. doi: 10.1016/0042-6822(77)90125-8. [DOI] [PubMed] [Google Scholar]
- KILHAM L., MOLONEY J. B. ASSOCIATION OF RAT VIRUS AND MOLONEY LEUKEMIA VIRUS IN TISSUES OF INOCULATED RATS. J Natl Cancer Inst. 1964 Feb;32:523–531. [PubMed] [Google Scholar]
- Koczot F. J., Carter B. J., Garon C. F., Rose J. A. Self-complementarity of terminal sequences within plus or minus strands of adenovirus-associated virus DNA. Proc Natl Acad Sci U S A. 1973 Jan;70(1):215–219. doi: 10.1073/pnas.70.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra S. DNA replication in viruses. Annu Rev Genet. 1980;14:347–397. doi: 10.1146/annurev.ge.14.120180.002023. [DOI] [PubMed] [Google Scholar]
- Mitra S., Snyder C. E., Bates R. C., Banerjee P. T. Comparative physicochemical and biological properties of two strains of Kilham rat virus, a non-defective parvovirus. J Gen Virol. 1982 Jul;61(Pt 50):43–54. doi: 10.1099/0022-1317-61-1-43. [DOI] [PubMed] [Google Scholar]
- Muller D. E., Siegl G. Maturation of parvovirus LuIII in a subcellular system. I. Optimal conditions for in vitro synthesis and encapsidation of viral DNA. J Gen Virol. 1983 May;64(Pt 5):1043–1054. doi: 10.1099/0022-1317-64-5-1043. [DOI] [PubMed] [Google Scholar]
- Revie D., Tseng B. Y., Grafstrom R. H., Goulian M. Covalent association of protein with replicative form DNA of parvovirus H-1. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5539–5543. doi: 10.1073/pnas.76.11.5539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode S. L., 3rd Replication process of the parvovirus H-1. X. Isolation of a mutant defective in replicative-form DNA replication. J Virol. 1978 Jan;25(1):215–223. doi: 10.1128/jvi.25.1.215-223.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegl G. Physicochemical characteristics of the DNA of parvovirus Lu 3. Arch Gesamte Virusforsch. 1973;43(4):334–344. doi: 10.1007/BF01556150. [DOI] [PubMed] [Google Scholar]