Abstract
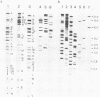
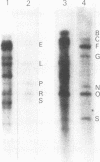
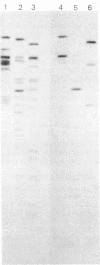
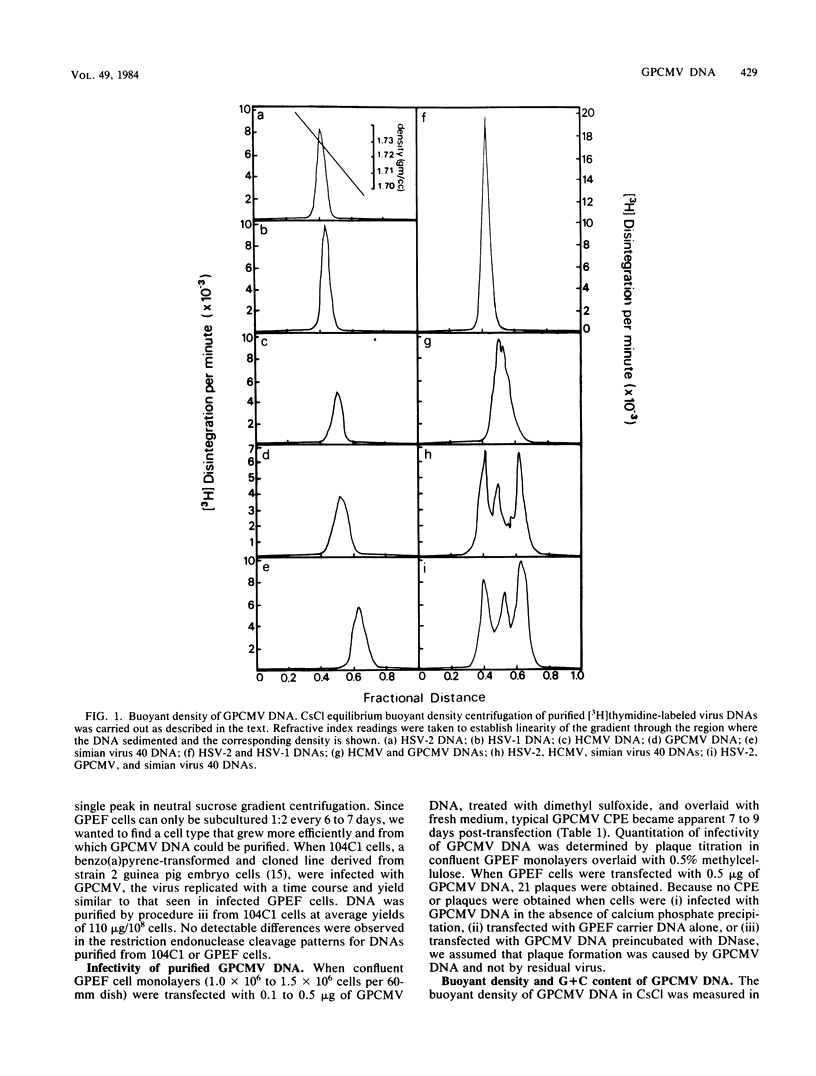
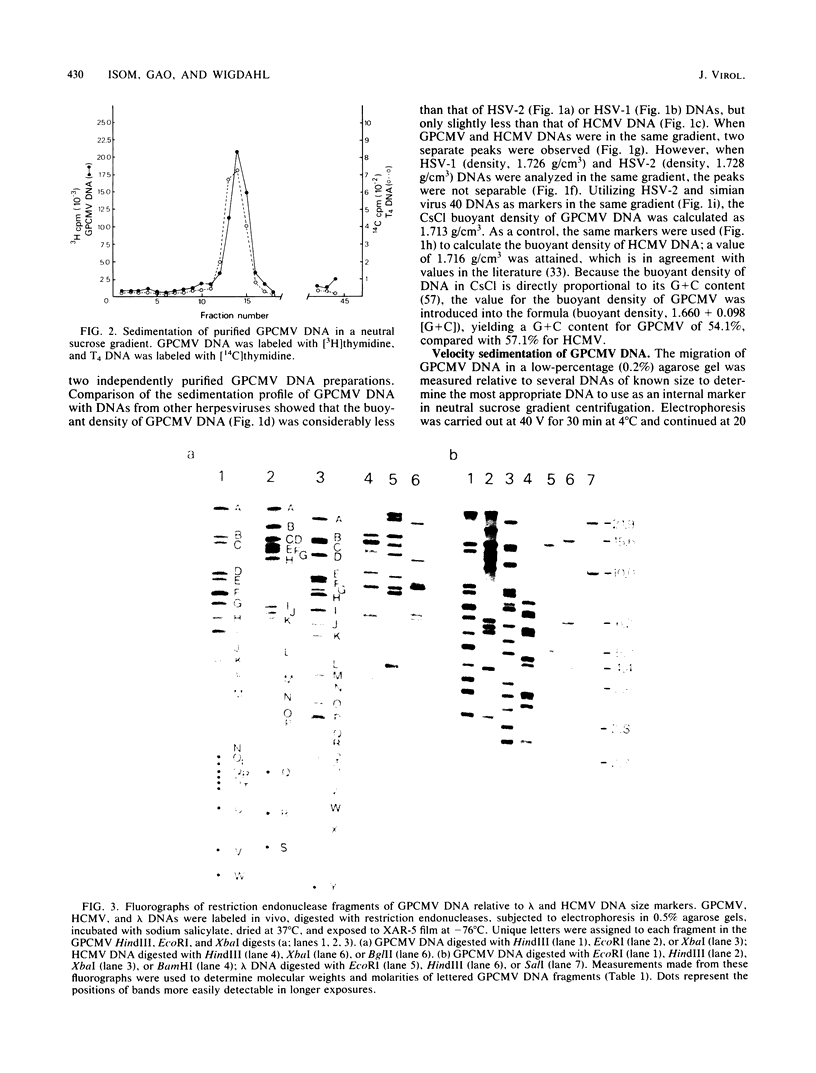
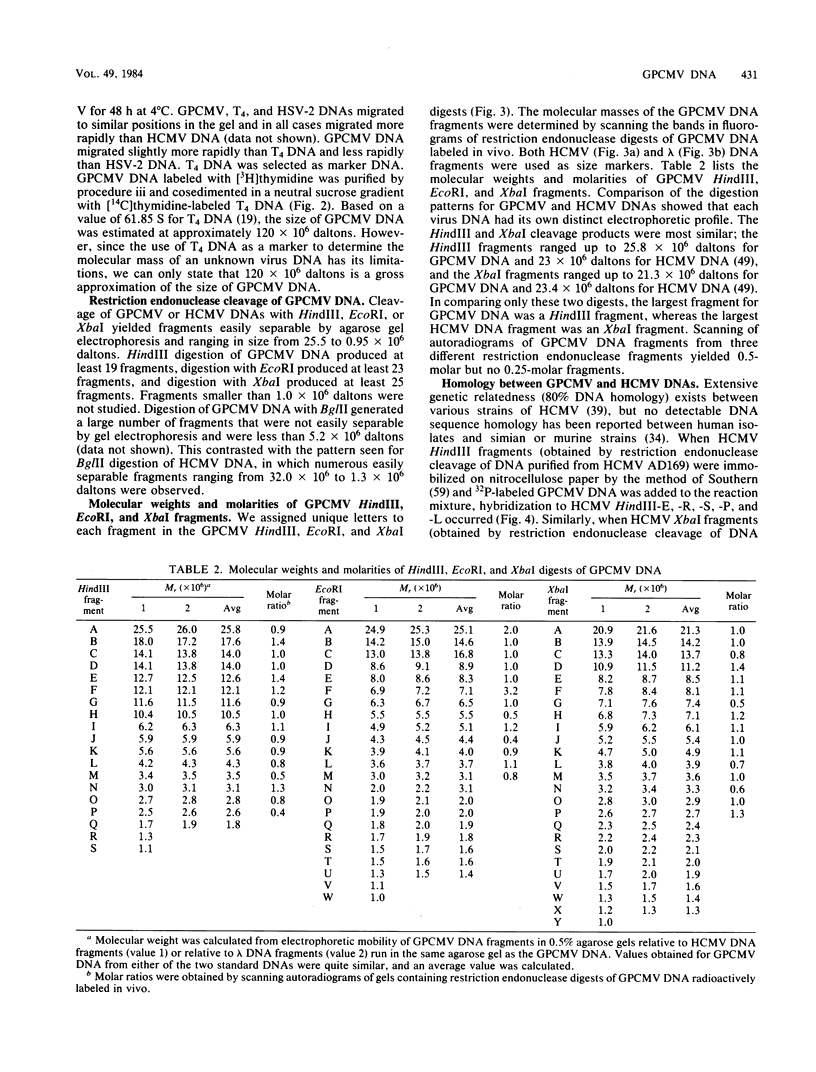
The genome of guinea pig cytomegalovirus (GPCMV) was analyzed and compared with that of human cytomegalovirus (HCMV). GPCMV and HCMV DNAs were isolated from virions and further purified by CsCl centrifugation. Purified GPCMV DNA sedimented as a single peak in a neutral sucrose gradient and was infectious when transfected into guinea pig embryo fibroblast cells. The cytopathology was characteristic of that seen after infection with GPCMV. Virus DNA purified from virions isolated from infected GPEF or 104C1 cells had a CsCl buoyant density of 1.713 g/cm3, which corresponds to a guanine plus cytosine content of 54.1%. The CsCl buoyant density of GPCMV DNA was slightly less than that of HCMV DNA (1.716 g/cm3), but sufficiently different so that the two virus DNA peaks did not coincide. GPCMV DNA cosedimented with T4 DNA in a neutral sucrose gradient. Restriction endonuclease cleavage of GPCMV or HCMV DNAs with HindIII, XbaI, or EcoRI yielded fragments easily separable by agarose gel electrophoresis and ranging from 1.0 X 10(6) to 25.8 X 10(6) daltons. The number, size, and molarity of GPCMV DNA fragments generated by restriction enzymes were determined. Hybridization of restriction endonuclease-cleaved GPCMV DNA with radioactively labeled HCMV DNA and, conversely, hybridization of restriction endonuclease-cleaved HCMV DNA with radioactively labeled GPCMV DNA indicated sequence homology between the two virus DNAs.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrecht T., Rapp F. Malignant transformation of hamster embryo fibroblasts following exposure to ultraviolet-irradiated human cytomegalovirus. Virology. 1973 Sep;55(1):53–61. doi: 10.1016/s0042-6822(73)81007-4. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bia F. J., Hastings K., Hsiung G. D. Cytomegalovirus infection in guinea pigs. III. Persistent viruria, blood transmission, and viral interference. J Infect Dis. 1979 Dec;140(6):914–920. doi: 10.1093/infdis/140.6.914. [DOI] [PubMed] [Google Scholar]
- Bia F. J., Summers W. C., Fong C. K., Hsiung G. D. New endogenous herpesvirus of guinea pigs: biological and molecular characterization. J Virol. 1980 Oct;36(1):245–253. doi: 10.1128/jvi.36.1.245-253.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldogh I., Gönczöl E., Gärtner L., Váczi G. Expression of the human cytomegalovirus genome in mouse cells and in human-mouse heterokaryons. Arch Virol. 1977;53(1-2):101–108. doi: 10.1007/BF01314851. [DOI] [PubMed] [Google Scholar]
- Boldogh I., Gönczöl E., Váczi L. Transformation of hamster embryonic fibroblast cells by UV-irradiated human cytomegalovirus. Acta Microbiol Acad Sci Hung. 1978;25(4):269–275. [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Choi Y. C., Hsiung G. D. Cytomegalovirus infection in guinea pigs. II. Transplacental and horizontal transmission. J Infect Dis. 1978 Aug;138(2):197–202. doi: 10.1093/infdis/138.2.197. [DOI] [PubMed] [Google Scholar]
- Colberg-Poley A. M., Isom H. C., Rapp F. Involvement of an early human cytomegalovirus function in reactivation of quiescent herpes simplex virus type 2. J Virol. 1981 Mar;37(3):1051–1059. doi: 10.1128/jvi.37.3.1051-1059.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMarchi J. M., Blankenship M. L., Brown G. D., Kaplan A. S. Size and complexity of human cytomegalovirus DNA. Virology. 1978 Sep;89(2):643–646. doi: 10.1016/0042-6822(78)90209-x. [DOI] [PubMed] [Google Scholar]
- DeMarchi J. M., Kaplan A. S. Physiological state of human embryonic lung cells affects their response to human cytomegalovirus. J Virol. 1977 Jul;23(1):126–132. doi: 10.1128/jvi.23.1.126-132.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durack D. T. Opportunistic infections and Kaposi's sarcoma in homosexual men. N Engl J Med. 1981 Dec 10;305(24):1465–1467. doi: 10.1056/NEJM198112103052408. [DOI] [PubMed] [Google Scholar]
- Ecker J. R., Hyman R. W. Varicella-zoster virus vaccine DNA differs from the parental virus DNA. J Virol. 1981 Oct;40(1):314–318. doi: 10.1128/jvi.40.1.314-318.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans C. H., DiPaolo J. A. Neoplastic transformation of guinea pig fetal cells in culture induced by chemical carcinogens. Cancer Res. 1975 Apr;35(4):1035–1044. [PubMed] [Google Scholar]
- Fenoglio C. M., Oster M. W., Lo Gerfo P., Reynolds T., Edelson R., Patterson J. A., Madeiros E., McDougall J. K. Kaposi's sarcoma following chemotherapy for testicular cancer in a homosexual man: demonstration of cytomegalovirus RNA in sarcoma cells. Hum Pathol. 1982 Oct;13(10):955–959. doi: 10.1016/s0046-8177(82)80063-4. [DOI] [PubMed] [Google Scholar]
- Fioretti A., Furukawa T., Santoli D., Plotkin S. A. Nonproductive infection of guinea pig cells with human cytomegalovirus. J Virol. 1973 Jun;11(6):998–1003. doi: 10.1128/jvi.11.6.998-1003.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freifelder D. Molecular weights of coliphages and coliphage DNA. IV. Molecular weights of DNA from bacteriophages T4, T5 and T7 and the general problem of determination of M. J Mol Biol. 1970 Dec 28;54(3):567–577. doi: 10.1016/0022-2836(70)90127-0. [DOI] [PubMed] [Google Scholar]
- Furukawa T., Fioretti A., Plotkin S. Growth characteristics of cytomegalovirus in human fibroblasts with demonstration of protein synthesis early in viral replication. J Virol. 1973 Jun;11(6):991–997. doi: 10.1128/jvi.11.6.991-997.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa T., Yoshimura N., Jean J. H., Plotkin S. A. Chronically persistent infection with human cytomegalovirus in human lymphoblasts. J Infect Dis. 1979 Feb;139(2):211–214. doi: 10.1093/infdis/139.2.211. [DOI] [PubMed] [Google Scholar]
- Färber I., Wutzler P., Schweizer H., Sprössig M. Human cytomegalovirus induced changes in rabbit cells. Brief report. Arch Virol. 1979;59(3):257–261. doi: 10.1007/BF01317421. [DOI] [PubMed] [Google Scholar]
- Geder K. M., Lausch R., O'Neill F., Rapp F. Oncogenic transformation of human embryo lung cells by human cytomegalovirus. Science. 1976 Jun 11;192(4244):1134–1137. doi: 10.1126/science.179143. [DOI] [PubMed] [Google Scholar]
- Geder L., Rapp F. Herpesviruses and prostate carcinogenesis. Arch Androl. 1980 Feb;4(1):71–78. doi: 10.3109/01485018008988282. [DOI] [PubMed] [Google Scholar]
- Geelen J. L., Walig C., Wertheim P., van der Noordaa J. Human cytomegalovirus DNA. I. Molecular weight and infectivity. J Virol. 1978 Jun;26(3):813–816. doi: 10.1128/jvi.26.3.813-816.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraldo G., Beth E., Henle W., Henle G., Mike V., Safai B., Huraux J. M., McHardy J., deThé G. Antibody patterns to herpesviruses in Kaposi's sarcoma. II. Serological association of American Kaposi's sarcoma with cytomegalovirus. Int J Cancer. 1978 Aug 15;22(2):126–131. doi: 10.1002/ijc.2910220204. [DOI] [PubMed] [Google Scholar]
- Giraldo G., Beth E., Huang E. S. Kaposi's sarcoma and its relationship to cytomegalovirus (CMNV). III. CMV DNA and CMV early antigens in Kaposi's sarcoma. Int J Cancer. 1980 Jul 15;26(1):23–29. doi: 10.1002/ijc.2910260105. [DOI] [PubMed] [Google Scholar]
- Griffith B. P., Lucia H. L., Bia F. J., Hsiung G. D. Cytomegalovirus-induced mononucleosis in guinea pigs. Infect Immun. 1981 May;32(2):857–863. doi: 10.1128/iai.32.2.857-863.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanshaw J. B. Congenital cytomegalovirus infection: a fifteen year perspective. J Infect Dis. 1971 May;123(5):555–561. doi: 10.1093/infdis/123.5.555. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Hsiung G. D., Choi Y. C., Bia F. Cytomegalovirus infection in guinea pigs. I. Viremia during acute primary and chronic persistent infection. J Infect Dis. 1978 Aug;138(2):191–196. doi: 10.1093/infdis/138.2.191. [DOI] [PubMed] [Google Scholar]
- Hsiung G. D., Tenser R. B., Fong C. K. Comparison of guinea pig cytomegalovirus and guinea pig herpes-like virus: growth characteristics and antigentic relationship. Infect Immun. 1976 Mar;13(3):926–933. doi: 10.1128/iai.13.3.926-933.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang E. S., Chen S. T., Pagano J. S. Human cytomegalovirus. I. Purification and characterization of viral DNA. J Virol. 1973 Dec;12(6):1473–1481. doi: 10.1128/jvi.12.6.1473-1481.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang E. S., Pagano J. S. Human cytomegalovirus. II. Lack of relatedness to DNA of herpes simples I and II, Epstein-Barr virus, and nonhuman strains of cytomegalovirus. J Virol. 1974 Mar;13(3):642–645. doi: 10.1128/jvi.13.3.642-645.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang E. S., Roche J. K. Cytomegalovirus D.N.A. and adenocarcinoma of the colon: Evidence for latent viral infection. Lancet. 1978 May 6;1(8071):957–960. doi: 10.1016/s0140-6736(78)90248-9. [DOI] [PubMed] [Google Scholar]
- Isom H. C., Mummaw J., Kreider J. W. Malignant transformation of guinea pig cells after exposure to ultraviolet-irradiated guinea pig cytomegalovirus. Virology. 1983 Apr 30;126(2):693–700. doi: 10.1016/s0042-6822(83)80025-7. [DOI] [PubMed] [Google Scholar]
- Johnson R., Horwitz S. N., Frost P. Disseminated Kaposi's sarcoma in a homosexual man. JAMA. 1982 Mar 26;247(12):1739–1741. [PubMed] [Google Scholar]
- Kilpatrick B. A., Huang E. S. Human cytomegalovirus genome: partial denaturation map and organization of genome sequences. J Virol. 1977 Oct;24(1):261–276. doi: 10.1128/jvi.24.1.261-276.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick B. A., Huang E. S., Pagano J. S. Analysis of cytomegalovirus genomes with restriction endonucleases Hin D III and EcoR-1. J Virol. 1976 Jun;18(3):1095–1105. doi: 10.1128/jvi.18.3.1095-1105.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M. L., Nankervis G. A. Experimental congenital infection with cytomegalovirus: a guinea pig model. J Infect Dis. 1978 Nov;138(5):650–654. doi: 10.1093/infdis/138.5.650. [DOI] [PubMed] [Google Scholar]
- Lakeman A. D., Osborn J. E. Size of infectious DNA from human and murine cytomegaloviruses. J Virol. 1979 Apr;30(1):414–416. doi: 10.1128/jvi.30.1.414-416.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonough S. H., Spector D. H. Transcription in human fibroblasts permissively infected by human cytomegalovirus strain AD169. Virology. 1983 Feb;125(1):31–46. doi: 10.1016/0042-6822(83)90061-2. [DOI] [PubMed] [Google Scholar]
- Melnick J. L., Lewis R., Wimberly I., Kaufman R. H., Adam E. Association of cytomegalovirus (CMV) infection with cervical cancer: isolation of CMV from cell cultures derived from cervical biopsy. Intervirology. 1978;10(2):115–119. doi: 10.1159/000148975. [DOI] [PubMed] [Google Scholar]
- Mocarski E. S., Stinski M. F. Persistence of the cytomegalovirus genome in human cells. J Virol. 1979 Sep;31(3):761–775. doi: 10.1128/jvi.31.3.761-775.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. R., Hudson J. B. Some properties of the genome of murine cytomegalovirus (MCV). Virology. 1973 Jul;54(1):135–149. doi: 10.1016/0042-6822(73)90123-2. [DOI] [PubMed] [Google Scholar]
- Neiman P. E., Reeves W., Ray G., Flournoy N., Lerner K. G., Sale G. E., Thomas E. D. A prospective analysis interstitial pneumonia and opportunistic viral infection among recipients of allogeneic bone marrow grafts. J Infect Dis. 1977 Dec;136(6):754–767. doi: 10.1093/infdis/136.6.754. [DOI] [PubMed] [Google Scholar]
- Nelson J. A., Fleckenstein B., Galloway D. A., McDougall J. K. Transformation of NIH 3T3 cells with cloned fragments of human cytomegalovirus strain AD169. J Virol. 1982 Jul;43(1):83–91. doi: 10.1128/jvi.43.1.83-91.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oram J. D., Downing R. G., Akrigg A., Dollery A. A., Duggleby C. J., Wilkinson G. W., Greenaway P. J. Use of recombinant plasmids to investigate the structure of the human cytomegalovirus genome. J Gen Virol. 1982 Mar;59(Pt 1):111–129. doi: 10.1099/0022-1317-59-1-111. [DOI] [PubMed] [Google Scholar]
- Pater M. M., Hyman R. W., Rapp F. Isolation of herpes simplex virus DNA from the "hirt supernatant". Virology. 1976 Dec;75(2):481–483. doi: 10.1016/0042-6822(76)90046-5. [DOI] [PubMed] [Google Scholar]
- Rapp F., Geder L., Murasko D., Lausch R., Ladda R., Huang E. S., Webber M. M. Long-term persistence of cytomegalovirus genome in cultured human cells of prostatic origin. J Virol. 1975 Oct;16(4):982–990. doi: 10.1128/jvi.16.4.982-990.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards G. M. Modifications of the diphenylamine reaction giving increased sensitivity and simplicity in the estimation of DNA. Anal Biochem. 1974 Feb;57(2):369–376. doi: 10.1016/0003-2697(74)90091-8. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rubin R. H., Cosimi A. B., Tolkoff-Rubin N. E., Russell P. S., Hirsch M. S. Infectious disease syndromes attributable to cytomegalovirus and their significance among renal transplant recipients. Transplantation. 1977 Dec;24(6):458–464. doi: 10.1097/00007890-197712000-00010. [DOI] [PubMed] [Google Scholar]
- SCHILDKRAUT C. L., MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its buoyant density in CsCl. J Mol Biol. 1962 Jun;4:430–443. doi: 10.1016/s0022-2836(62)80100-4. [DOI] [PubMed] [Google Scholar]
- Sanford E. J., Dagen J. E., Geder L., Rohner T. J., Jr, Rapp F. Lymphocyte reactivity against virally transformed cells in patients with urologic cancer. J Urol. 1977 Nov;118(5):809–810. doi: 10.1016/s0022-5347(17)58203-8. [DOI] [PubMed] [Google Scholar]
- Sanford E. J., Geder L., Dagen J. E., Laychock A., Rohner T. J., Jr, Rapp F. Humoral and cellular immune response of prostatic cancer patients to cytomegalovirus-related antigens. J Surg Res. 1978 May;24(5):404–408. doi: 10.1016/0022-4804(78)90035-5. [DOI] [PubMed] [Google Scholar]
- Sokol F., Kuwert E., Wiktor T. J., Hummeler K., Koprowski H. Purification of rabies virus grown in tissue culture. J Virol. 1968 Aug;2(8):836–849. doi: 10.1128/jvi.2.8.836-849.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spector D. H., Hock L., Tamashiro J. C. Cleavage maps for human cytomegalovirus DNA strain AD169 for restriction endonucleases EcoRI, BglII, and HindIII. J Virol. 1982 May;42(2):558–582. doi: 10.1128/jvi.42.2.558-582.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector D. J. Transcription of adenovirus 5 early region 1b is elevated in permissive cells infected by a mutant with an upstream deletion. J Virol. 1982 Nov;44(2):544–554. doi: 10.1128/jvi.44.2.544-554.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinski M. F., Mocarski E. S., Thomsen D. R. DNA of human cytomegalovirus: size heterogeneity and defectiveness resulting from serial undiluted passage. J Virol. 1979 Jul;31(1):231–239. doi: 10.1128/jvi.31.1.231-239.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenser R. B., Hsiung G. D. Comparison of guinea pig cytomegalovirus and guinea pig herpes-like virus: pathogenesis and persistence in experimentally infected animals. Infect Immun. 1976 Mar;13(3):934–940. doi: 10.1128/iai.13.3.934-940.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen D. R., Stinski M. F. Cloning of the human cytomegalovirus genome as endonuclease XbaI fragments. Gene. 1981 Dec;16(1-3):207–216. doi: 10.1016/0378-1119(81)90077-9. [DOI] [PubMed] [Google Scholar]
- Tyms A. S. Diseases of the fetus and neonate due to human cytomegalovirus: a laboratory perspective. Med Lab Sci. 1982 Jul;39(3):275–286. [PubMed] [Google Scholar]
- Weller T. H. The cytomegaloviruses: ubiquitous agents with protean clinical manifestations. I. N Engl J Med. 1971 Jul 22;285(4):203–214. doi: 10.1056/NEJM197107222850406. [DOI] [PubMed] [Google Scholar]
- Wharton J. H., Henry B. E., O'Callaghan D. J. Equine cytomegalovirus: cultural characteristics and properties of viral DNA. Virology. 1981 Feb;109(1):106–119. doi: 10.1016/0042-6822(81)90475-x. [DOI] [PubMed] [Google Scholar]
- Wigdahl B. L., Isom H. C., De Clercq E., Rapp F. Activation of herpes simplex virus (HSV) type 1 genome by temperature-sensitive mutants of HSV type 2. Virology. 1982 Jan 30;116(2):468–479. doi: 10.1016/0042-6822(82)90140-4. [DOI] [PubMed] [Google Scholar]
- Wigdahl B. L., Isom H. C., Rapp F. Repression and activation of the genome of herpes simplex viruses in human cells. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6522–6526. doi: 10.1073/pnas.78.10.6522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yourtee E. L., Bia F. J., Griffith B. P., Root R. K. Neutrophil response and function during acute cytomegalovirus infection in guinea pigs. Infect Immun. 1982 Apr;36(1):11–16. doi: 10.1128/iai.36.1.11-16.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]