Abstract
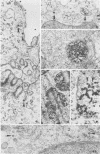
Simian virus 40 (SV40), which transforms mouse cells in vitro, has not been previously observed to cause tumors when injected in immunocompetent mice. We have investigated both the fate of the injected virion in mice and several immunological parameters as potential factors controlling tumorigenicity. We find that although SV40 does not replicate in mouse cells, the viral DNA can persist for many months postinjection; the majority of the viral DNA is found in the cytoplasm, but a small amount of the viral DNA is integrated at multiple sites in the host nuclear DNA. The persistence of the viral genome is independent of the ability of the mouse to mount an SV40 TSTA specific cytotoxic T-cell response and may be attributed to the cytoplasmic location of the majority of the viral genome. However, in long-term studies of SV40-injected mice, genetically identical except for the major histocompatibility complex, we find that tumors were induced in some mice of the H-2d (low cytotoxic T-lymphocyte responder to SV40 TSTA) but not of the H-2k (high responder to SV40 TSTA) haplotype. Thus, a combination of inefficient disposal of the injected virion and inefficient immunological surveillance and elimination of cells containing nuclear SV40 DNA can eventually result in SV40-induced tumors at multiple sites in mice.
Full text
PDF


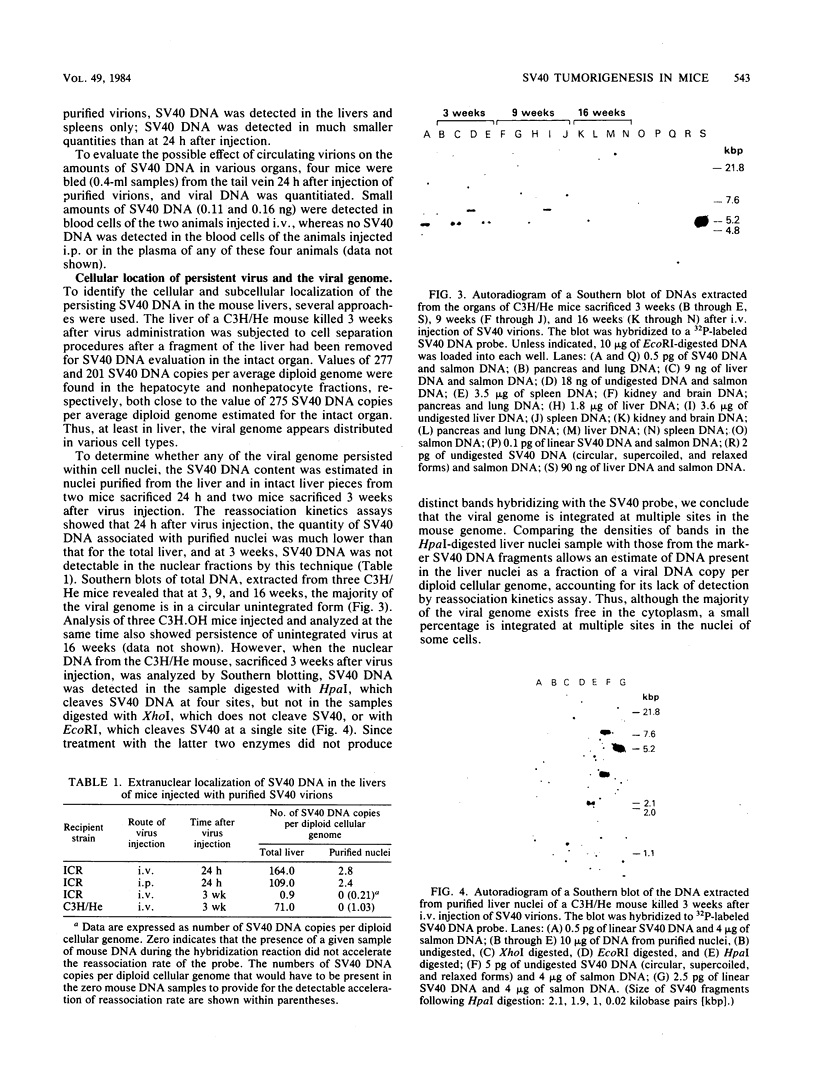


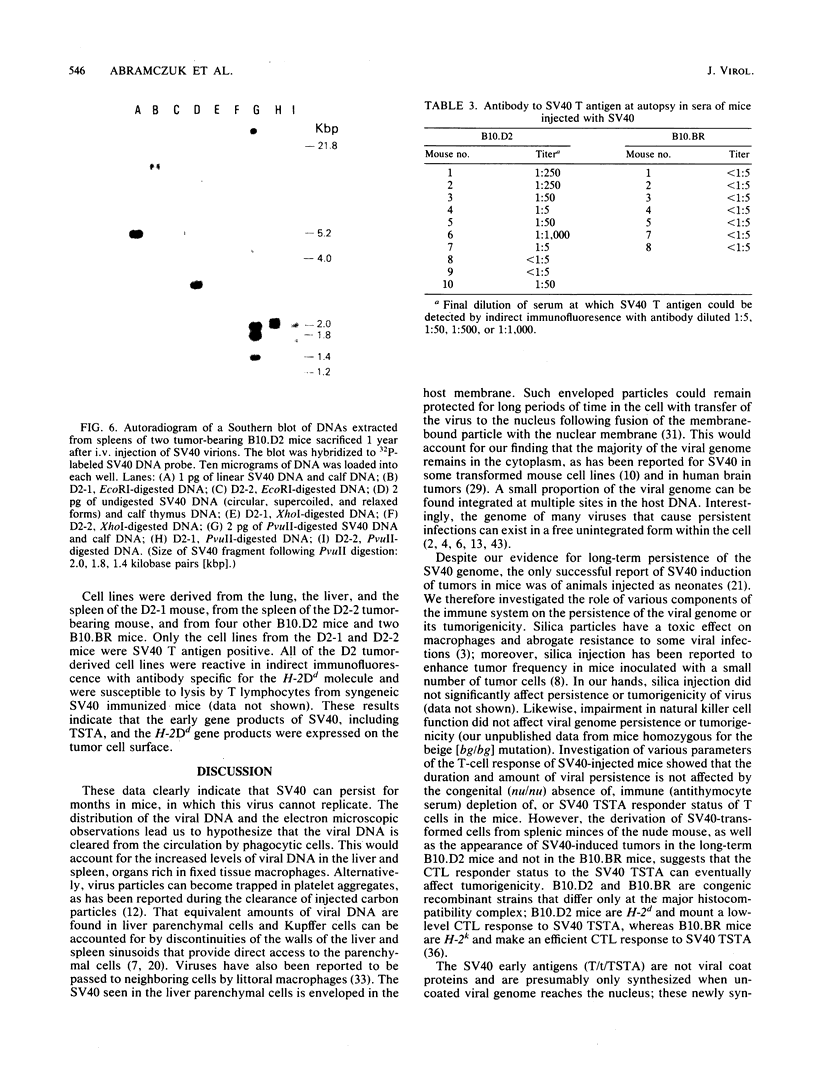


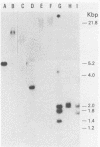
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Todaro G. J. Basis for the acquisition of malignant potential by mouse cells cultivated in vitro. Science. 1968 Nov 29;162(3857):1024–1026. doi: 10.1126/science.162.3857.1024. [DOI] [PubMed] [Google Scholar]
- Adams A., Lindahl T. Epstein-Barr virus genomes with properties of circular DNA molecules in carrier cells. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1477–1481. doi: 10.1073/pnas.72.4.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amtmann E., Müller H., Sauer G. Equine connective tissue tumors contain unintegrated bovine papilloma virus DNA. J Virol. 1980 Sep;35(3):962–964. doi: 10.1128/jvi.35.3.962-964.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Potter V. R. Nuclei from rat liver: isolation method that combines purity with high yield. Science. 1966 Dec 30;154(3757):1662–1665. doi: 10.1126/science.154.3757.1662. [DOI] [PubMed] [Google Scholar]
- Bréchot C., Hadchouel M., Scotto J., Fonck M., Potet F., Vyas G. N., Tiollais P. State of hepatitis B virus DNA in hepatocytes of patients with hepatitis B surface antigen-positive and -negative liver diseases. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3906–3910. doi: 10.1073/pnas.78.6.3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casley-Smith J. R., Reade P. C. An electron microscopical study of the uptake of foreign particles by the livers of foetal and adult rats. Br J Exp Pathol. 1965 Oct;46(5):473–480. [PMC free article] [PubMed] [Google Scholar]
- Chow D. A., Greene M. I., Greenberg A. H. Macrophage-dependent, NK-cell-independent "natural" surveillance of tumors in syngeneic mice. Int J Cancer. 1979 Jun 15;23(6):788–797. doi: 10.1002/ijc.2910230609. [DOI] [PubMed] [Google Scholar]
- Clark J. M., Pateman J. A. Long-term culture of Chinese hamster Kupffer cell lines isolated by a primary cloning step. Exp Cell Res. 1978 Mar 1;112(1):207–217. doi: 10.1016/0014-4827(78)90541-4. [DOI] [PubMed] [Google Scholar]
- Daya-Grosjean L., Monier R. Presence of free viral DNA in simian virus 40-transformed nonproducer cells. J Virol. 1978 Aug;27(2):307–312. doi: 10.1128/jvi.27.2.307-312.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Donal K. J. The role of platelets in the clearance of colloidal carbon from blood in rabbits: a light and electron microscope study. Pathology. 1972 Oct;4(4):295–301. doi: 10.3109/00313027209068955. [DOI] [PubMed] [Google Scholar]
- Fraser N. W., Lawrence W. C., Wroblewska Z., Gilden D. H., Koprowski H. Herpes simplex type 1 DNA in human brain tissue. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6461–6465. doi: 10.1073/pnas.78.10.6461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann T., Haas M. Rapid concentration and purification of polyoma virus and SV40 with polyethylene glycol. Virology. 1970 Sep;42(1):248–250. doi: 10.1016/0042-6822(70)90263-1. [DOI] [PubMed] [Google Scholar]
- Gelb L. D., Kohne D. E., Martin M. A. Quantitation of Simian virus 40 sequences in African green monkey, mouse and virus-transformed cell genomes. J Mol Biol. 1971 Apr 14;57(1):129–145. doi: 10.1016/0022-2836(71)90123-9. [DOI] [PubMed] [Google Scholar]
- Gooding L. R. Characterization of a progressive tumor from C3H fibroblasts transformed in vitro with SV40 virus. Immunoresistance in vivo correlates with phenotypic loss of H-2Kk. J Immunol. 1982 Sep;129(3):1306–1312. [PubMed] [Google Scholar]
- Gooding L. R. Specificities of killing by T lymphocytes generated against syngeneic SV40 transformants: studies employing recombinants within the H-2 complex. J Immunol. 1979 Mar;122(3):1002–1008. [PubMed] [Google Scholar]
- Gooding L. R. Specificities of killing by cytotoxic lymphocytes generated in vivo and in vitro to syngeneic SV40 transformed cells. J Immunol. 1977 Mar;118(3):920–927. [PubMed] [Google Scholar]
- Gross-Bellard M., Oudet P., Chambon P. Isolation of high-molecular-weight DNA from mammalian cells. Eur J Biochem. 1973 Jul 2;36(1):32–38. doi: 10.1111/j.1432-1033.1973.tb02881.x. [DOI] [PubMed] [Google Scholar]
- HAMPTON J. C. An electron microscope study of the hepatic uptake and excretion of submicroscopic particles injected into the blood stream and into the bile duct. Acta Anat (Basel) 1958;32(3):262–291. doi: 10.1159/000141328. [DOI] [PubMed] [Google Scholar]
- Hargis B. J., Malkiel S. Sarcomas induced by injection of simian virus 40 into neonatal CFW mice. J Natl Cancer Inst. 1979 Oct;63(4):965–968. [PubMed] [Google Scholar]
- Hölzel F., Sokol F. Integration of progeny simian virus 40 DNA into the host cell genome. J Mol Biol. 1974 Apr 15;84(3):423–444. doi: 10.1016/0022-2836(74)90450-1. [DOI] [PubMed] [Google Scholar]
- Israel M. A., Martin M. A., Takemoto K. K., Howley P. M., Aaronson S. A., Solomon D., Khoury G. Evaluation of normal and neoplastic human tissue for BK virus. Virology. 1978 Oct 15;90(2):187–196. doi: 10.1016/0042-6822(78)90302-1. [DOI] [PubMed] [Google Scholar]
- Jaenisch R., Mintz B. Simian virus 40 DNA sequences in DNA of healthy adult mice derived from preimplantation blastocysts injected with viral DNA. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1250–1254. doi: 10.1073/pnas.71.4.1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kit S., Kurimura T., Dubbs D. R. Transplantable mouse tumor line induced by injection of SV40-transformed mouse kidney cells. Int J Cancer. 1969 Jul 15;4(4):384–392. doi: 10.1002/ijc.2910040403. [DOI] [PubMed] [Google Scholar]
- Knowles B. B., Ford S. R., Aden D. P. Reactivity to SV40 T antigen in athymic (nude), anti-thymocyte serum-treated, and normal mice. J Immunol. 1977 Jul;119(1):79–82. [PubMed] [Google Scholar]
- Knowles B. B., Koncar M., Pfizenmaier K., Solter D., Aden D. P., Trinchieri G. Genetic control of the cytotoxic T cell response to SV40 tumor-associated specific antigen. J Immunol. 1979 May;122(5):1798–1806. [PubMed] [Google Scholar]
- Krieg P., Amtmann E., Jonas D., Fischer H., Zang K., Sauer G. Episomal simian virus 40 genomes in human brain tumors. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6446–6450. doi: 10.1073/pnas.78.10.6446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIMS C. A. ASPECTS OF THE PATHOGENESIS OF VIRUS DISEASES. Bacteriol Rev. 1964 Mar;28:30–71. doi: 10.1128/br.28.1.30-71.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maul G. G., Rovera G., Vorbrodt A., Abramczuk J. Membrane fusion as a mechanism of simian virus 40 entry into different cellular compartments. J Virol. 1978 Dec;28(3):936–944. doi: 10.1128/jvi.28.3.936-944.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland V. W., Mora P. T., Schultz A., Pancake S. Cell properties after repeated transplantation of spontaneously and of SV40 virus transformed mouse cell lines. I. Growth in culture. J Cell Physiol. 1975 Feb;85(1):101–111. doi: 10.1002/jcp.1040850111. [DOI] [PubMed] [Google Scholar]
- POPE J. H., ROWE W. P. DETECTION OF SPECIFIC ANTIGEN IN SV40-TRANSFORMED CELLS BY IMMUNOFLUORESCENCE. J Exp Med. 1964 Aug 1;120:121–128. doi: 10.1084/jem.120.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan S., Knowles B. B. Monoclonal antibody to SV40 T-antigen blocks lysis of cloned cytotoxic T-cell line specific for SV40 TASA. Virology. 1983 Feb;125(1):1–7. doi: 10.1016/0042-6822(83)90058-2. [DOI] [PubMed] [Google Scholar]
- Pan S., Wettstein P. J., Knowles B. B. H-2Kb mutations limit the CTL response to SV40 TASA. J Immunol. 1982 Jan;128(1):243–246. [PubMed] [Google Scholar]
- Pfizenmaier K., Pan S. H., Knowles B. B. Preferential H-2 association in cytotoxic T cell responses to SV40 tumor-associated specific antigens. J Immunol. 1980 Apr;124(4):1888–1891. [PubMed] [Google Scholar]
- Pfizenmaier K., Trinchieri G., Solter D., Knowles B. B. Mapping of H-2 genes associated with T cell-mediated cytotoxic responses to SV40-tumour-associated specific antigens. Nature. 1978 Aug 17;274(5672):691–693. doi: 10.1038/274691a0. [DOI] [PubMed] [Google Scholar]
- Robb J. A., Martin R. G. Genetic analysis of simian virus 40. I. Description of microtitration and replica-plating techniques for virus. Virology. 1970 Aug;41(4):751–760. doi: 10.1016/0042-6822(70)90439-3. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Pettersson U., Sambrook J. Viral DNA in transformed cells. I. A study of the sequences of adenovirus 2 DNA in a line of transformed rat cells using specific fragments of the viral genome. J Mol Biol. 1974 Jul 15;86(4):709–726. doi: 10.1016/0022-2836(74)90348-9. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Takemoto K. K., Linke H., Miyamura T., Fareed G. C. Persistent BK papovavirus infection of transformed human fetal brain cells. I. Episomal viral DNA in cloned lines deficient in T-antigen expression. J Virol. 1979 Mar;29(3):1177–1185. doi: 10.1128/jvi.29.3.1177-1185.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto K. K., Ting R. C., Ozer H. L., Fabisch P. Establishment of a cell line from an inbred mouse strain for viral transformation studies: simian virus 40 transformation and tumor production. J Natl Cancer Inst. 1968 Dec;41(6):1401–1409. [PubMed] [Google Scholar]
- Tan K. B., Sokol F. Structural proteins of simian virus 40: phosphoproteins. J Virol. 1972 Nov;10(5):985–994. doi: 10.1128/jvi.10.5.985-994.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tevethia S. S., Blasecki J. W., Waneck G., Goldstein A. L. Requirement of thymus-derived theta-positive lymphocytes for rejection of DNA virus (SV 40) tumors in mice. J Immunol. 1974 Nov;113(5):1417–1423. [PubMed] [Google Scholar]
- Tevethia S. S., Flyer D. C., Tjian R. Biology of simian virus 40 (SV40) transplantation antigen (TrAg). VI. Mechanism of induction of SV40 transplantation immunity in mice by purified SV40 T antigen (D2 protein). Virology. 1980 Nov;107(1):13–23. doi: 10.1016/0042-6822(80)90268-8. [DOI] [PubMed] [Google Scholar]
- Tevethia S. S., MacMillan V. L. Acquisition of malignant properties by SV40-transformed mouse cells: relationship to type-C viral antigen expression. Intervirology. 1974;3(4):269–276. doi: 10.1159/000149763. [DOI] [PubMed] [Google Scholar]
- Tevethia S. S., Waneck G., Tevethia M. J. Immune response of athymic nude mice to papovavirus SV40 tumor-associated antigens. Int J Cancer. 1977 May 15;19(5):700–706. doi: 10.1002/ijc.2910190516. [DOI] [PubMed] [Google Scholar]
- Trinchieri G., Aden D. P., Knowles B. B. Cell-mediated cytotoxicity to SV40-specific tumour-associated antigens. Nature. 1976 May 27;261(5558):312–314. doi: 10.1038/261312a0. [DOI] [PubMed] [Google Scholar]
- Tsuchida N., Robin M. S., Green M. Viral RNA subunits in cells transformed by RNA tumor viruses. Science. 1972 Jun 30;176(4042):1418–1420. doi: 10.1126/science.176.4042.1418. [DOI] [PubMed] [Google Scholar]
- Urban J. L., Burton R. C., Holland J. M., Kripke M. L., Schreiber H. Mechanisms of syngeneic tumor rejection. Susceptibility of host-selected progressor variants to various immunological effector cells. J Exp Med. 1982 Feb 1;155(2):557–573. doi: 10.1084/jem.155.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesslén T. SV40-tumorigenesis in mouse. Acta Pathol Microbiol Scand B Microbiol Immunol. 1970;78(4):479–487. doi: 10.1111/j.1699-0463.1970.tb04331.x. [DOI] [PubMed] [Google Scholar]
- Wright P. W., Smith H. S., McCoy J. Tumorigenicity and antigenicity of mouse cells infected with Simian virus 40. I. Relationship of growth in vitro and in vivo in immunosuppressed and immunocompetent recipients. J Natl Cancer Inst. 1973 Sep;51(3):951–959. doi: 10.1093/jnci/51.3.951. [DOI] [PubMed] [Google Scholar]
- zur Hausen H., Schulte-Holthausen H., Wolf H., Dörries K., Egger H. Attempts to detect virus-specific DNA in human tumors. II. Nucleic acid hybridizations with complementary RNA of human herpes group viruses. Int J Cancer. 1974 May 15;13(5):657–664. doi: 10.1002/ijc.2910130510. [DOI] [PubMed] [Google Scholar]