Abstract
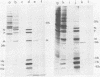
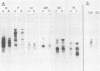
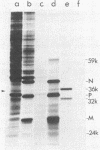
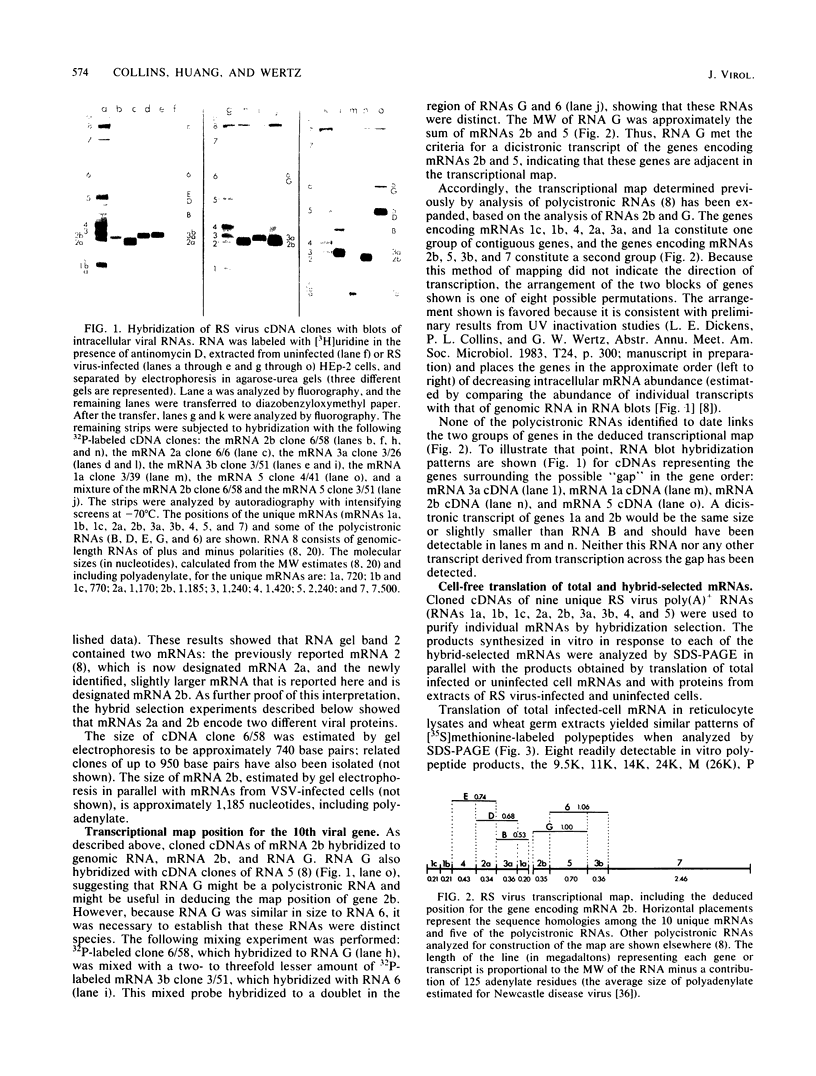
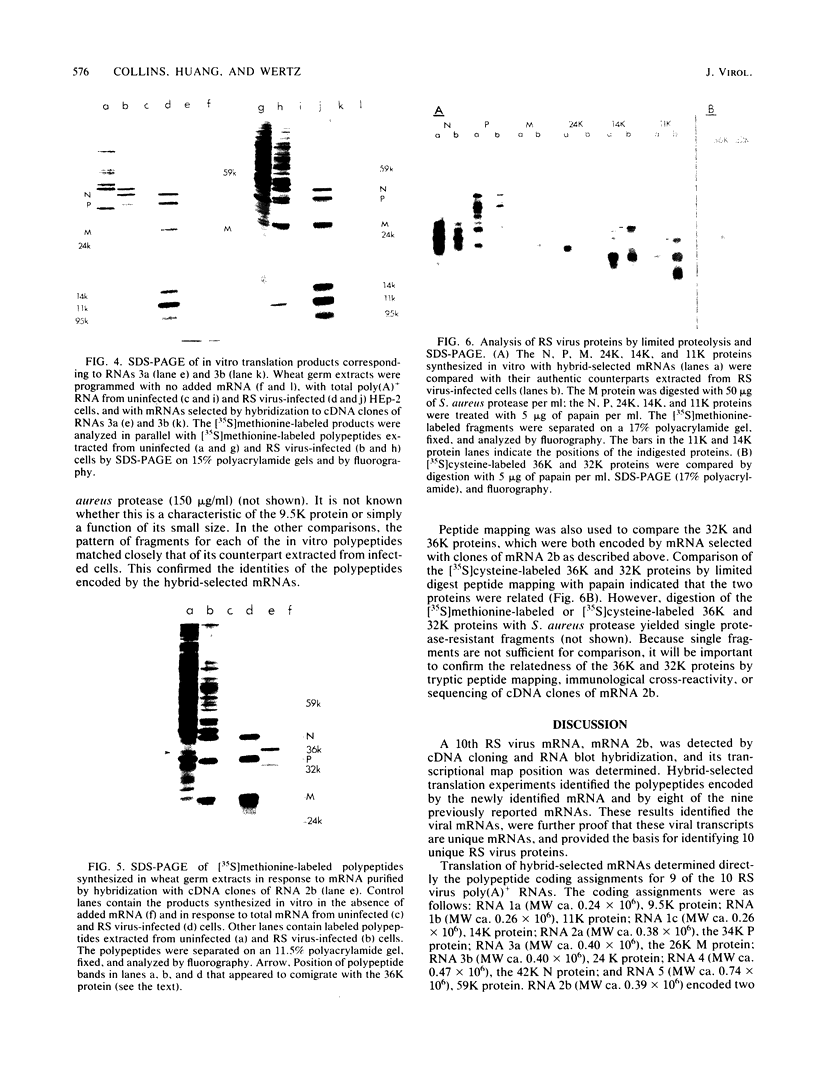
Nine mRNAs, their cDNA clones, and a genome transcriptional map have been reported previously for respiratory syncytial virus (P. L. Collins and G. W. Wertz, Proc. Natl. Acad. Sci. U.S.A. 80:3208-3212, 1983). We report here the identification of a 10th viral mRNA, designated mRNA 2b (molecular weight [MW] ca. 0.39 X 10(6)), that was detected by RNA (Northern) blot hybridization with cDNA clones. Analysis of a polycistronic readthrough transcript was used to deduce the position in the viral transcriptional map of the gene encoding the newly identified mRNA. The polypeptide coding assignments of 9 of the 10 respiratory syncytial virus mRNAs were determined. Individual viral mRNAs were purified by hybridization selection with nine unique, nonoverlapping cDNA clones and analyzed by translation in vitro. Each of the nine mRNAs encoded a single polypeptide chain. The coding assignments were as follows: RNA 1a (MW ca. 0.24 X 10(6)), a 9,500-dalton (9.5K) protein; RNA 1b (MW 0.26 X 10(6)), an 11K protein; RNA 1c (MW 0.26 X 10(6)), a 14K protein; RNA 2a (MW 0.38 X 10(6)), the 34K phosphorylated (P) protein; RNA 2b (MW 0.39 X 10(6)), a 36K protein; RNA 3a (MW 0.40 X 10(6)), the 26K matrix (M) protein; RNA 3b (MW 0.40 X 10(6)), a 24K protein; RNA 4 (MW 0.47 X 10(6)), the 42K major nucleocapsid (N) protein; and RNA 5 (MW 0.74 X 10(6)), a 59K protein. The cDNA clones used for the hybridization selections were respiratory syncytial virus specific and did not hybridize with uninfected-cell mRNA; therefore the proteins synthesized with the selected mRNAs were virus specific. The 9.5K, 11K, 14K, 24K, M, P, 36K, N, and 59K proteins were encoded by different mRNAs; therefore these nine proteins are all unique. The 9.5K, 11K, 14K, 24K, M, P, and N proteins synthesized in vitro with hybrid-selected mRNAs each had counterparts with the same electrophoretic mobilities in extracts of virus-infected cells. The in vitro polypeptides and their authentic counterparts were shown to be closely related by limited digest peptide mapping. The 36K and 59K polypeptides lacked counterparts with the same electrophoretic mobilities in infected cells and therefore are candidates for the unprocessed precursors of the viral F and G glycoproteins. The 10th viral mRNA, the 2,500K RNA 7, was not tested directly but is the only known mRNA of the appropriate size to encode the 200K large (L) protein of the viral nucleocapsid. These assignments account for all 10 of the reported viral mRNAs and bring to 10 the number of known unique viral proteins.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernstein J. M., Hruska J. F. Respiratory syncytial virus proteins: identification by immunoprecipitation. J Virol. 1981 Apr;38(1):278–285. doi: 10.1128/jvi.38.1.278-285.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash P., Pringle C. R., Preston C. M. The polypeptides of human respiratory syncytial virus: products of cell-free protein synthesis and post-translational modifications. Virology. 1979 Jan 30;92(2):375–384. doi: 10.1016/0042-6822(79)90142-9. [DOI] [PubMed] [Google Scholar]
- Cash P., Wunner W. H., Pringle C. R. A comparison of the polypeptides of human and bovine respiratory syncytial viruses and murine pneumonia virus. Virology. 1977 Oct 15;82(2):369–379. doi: 10.1016/0042-6822(77)90012-5. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Collins P. L., Hightower L. E., Ball L. A. Transcriptional map for Newcastle disease virus. J Virol. 1980 Sep;35(3):682–693. doi: 10.1128/jvi.35.3.682-693.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins P. L., Wertz G. W., Ball L. A., Hightower L. E. Coding assignments of the five smaller mRNAs of Newcastle disease virus. J Virol. 1982 Sep;43(3):1024–1031. doi: 10.1128/jvi.43.3.1024-1031.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins P. L., Wertz G. W. cDNA cloning and transcriptional mapping of nine polyadenylylated RNAs encoded by the genome of human respiratory syncytial virus. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3208–3212. doi: 10.1073/pnas.80.11.3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis N. L., Wertz G. W. Synthesis of vesicular stomatitis virus negative-strand RNA in vitro: dependence on viral protein synthesis. J Virol. 1982 Mar;41(3):821–832. doi: 10.1128/jvi.41.3.821-832.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubovi E. J. Analysis of proteins synthesized in respiratory syncytial virus-infected cells. J Virol. 1982 May;42(2):372–378. doi: 10.1128/jvi.42.2.372-378.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkind P. R., Cross R. K., Lamb R. A., Merz D. C., Choppin P. W. In vitro synthesis of structural and nonstructural proteins of Sendai and SV5 viruses. Virology. 1980 Jan 15;100(1):22–33. doi: 10.1016/0042-6822(80)90548-6. [DOI] [PubMed] [Google Scholar]
- Fernie B. F., Gerin J. L. Immunochemical identification of viral and nonviral proteins of the respiratory syncytial virus virion. Infect Immun. 1982 Jul;37(1):243–249. doi: 10.1128/iai.37.1.243-249.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazier K., Raghow R., Kingsbury D. W. Regulation of Sendai virus transcription: evidence for a single promoter in vivo. J Virol. 1977 Mar;21(3):863–871. doi: 10.1128/jvi.21.3.863-871.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall W. W., Lamb R. A., Choppin P. W. The polypeptides of canine distemper virus: synthesis in infected cells and relatedness to the polypeptides of other morbilliviruses. Virology. 1980 Jan 30;100(2):433–449. doi: 10.1016/0042-6822(80)90534-6. [DOI] [PubMed] [Google Scholar]
- Herman R. C., Adler S., Lazzarini R. A., Colonno R. J., Banerjee A. K., Westphal H. Intervening polyadenylate sequences in RNA transcripts of vesicular stomatitis virus. Cell. 1978 Oct;15(2):587–596. doi: 10.1016/0092-8674(78)90027-2. [DOI] [PubMed] [Google Scholar]
- Herman R. C., Schubert M., Keene J. D., Lazzarini R. A. Polycistronic vesicular stomatitis virus RNA transcripts. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4662–4665. doi: 10.1073/pnas.77.8.4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrler G., Compans R. W. Synthesis of mumps virus polypeptides in infected Vero cells. Virology. 1982 Jun;119(2):430–438. doi: 10.1016/0042-6822(82)90102-7. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Huang Y. T., Wertz G. W. Respiratory syncytial virus mRNA coding assignments. J Virol. 1983 May;46(2):667–672. doi: 10.1128/jvi.46.2.667-672.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y. T., Wertz G. W. The genome of respiratory syncytial virus is a negative-stranded RNA that codes for at least seven mRNA species. J Virol. 1982 Jul;43(1):150–157. doi: 10.1128/jvi.43.1.150-157.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter E., Hill E., Hardwick M., Bhown A., Schwartz D. E., Tizard R. Complete sequence of the Rous sarcoma virus env gene: identification of structural and functional regions of its product. J Virol. 1983 Jun;46(3):920–936. doi: 10.1128/jvi.46.3.920-936.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafatos F. C., Jones C. W., Efstratiadis A. Determination of nucleic acid sequence homologies and relative concentrations by a dot hybridization procedure. Nucleic Acids Res. 1979 Nov 24;7(6):1541–1552. doi: 10.1093/nar/7.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamb R. A., Choppin P. W. Determination by peptide mapping of the unique polypeptides in Sendai virions and infected cells. Virology. 1978 Feb;84(2):469–478. doi: 10.1016/0042-6822(78)90263-5. [DOI] [PubMed] [Google Scholar]
- Lambert D. M., Pons M. W., Mbuy G. N., Dorsch-Hasler K. Nucleic acids of respiratory syncytial virus. J Virol. 1980 Dec;36(3):837–846. doi: 10.1128/jvi.36.3.837-846.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine S. Polypeptides of respiratory syncytial virus. J Virol. 1977 Jan;21(1):427–431. doi: 10.1128/jvi.21.1.427-431.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeples M., Levine S. Respiratory syncytial virus polypeptides: their location in the virion. Virology. 1979 May;95(1):137–145. doi: 10.1016/0042-6822(79)90408-2. [DOI] [PubMed] [Google Scholar]
- Pringle C. R., Shirodaria P. V., Gimenez H. B., Levine S. Antigen and polypeptide synthesis by temperature-sensitive mutants of respiratory syncytial virus. J Gen Virol. 1981 May;54(Pt 1):173–183. doi: 10.1099/0022-1317-54-1-173. [DOI] [PubMed] [Google Scholar]
- Ricciardi R. P., Miller J. S., Roberts B. E. Purification and mapping of specific mRNAs by hybridization-selection and cell-free translation. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4927–4931. doi: 10.1073/pnas.76.10.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Ueba O. Purification and polypeptides of respiratory syncytial virus. Microbiol Immunol. 1980;24(4):361–364. doi: 10.1111/j.1348-0421.1980.tb02839.x. [DOI] [PubMed] [Google Scholar]
- Venkatesan S., Elango N., Chanock R. M. Construction and characterization of cDNA clones for four respiratory syncytial viral genes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1280–1284. doi: 10.1073/pnas.80.5.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh E. E., Hruska J. Monoclonal antibodies to respiratory syncytial virus proteins: identification of the fusion protein. J Virol. 1983 Jul;47(1):171–177. doi: 10.1128/jvi.47.1.171-177.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. R., Bratt M. A. Polyadenylate sequences on Newcastle disease virus mRNA synthesized in vivo and in vitro. J Virol. 1974 Jun;13(6):1220–1230. doi: 10.1128/jvi.13.6.1220-1230.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wunner W. H., Pringle C. R. Respiratory syncytial virus proteins. Virology. 1976 Aug;73(1):228–243. doi: 10.1016/0042-6822(76)90077-5. [DOI] [PubMed] [Google Scholar]