Abstract
Recycling of vesicles of the regulated secretory pathway presumably involves passage through an early endosomal compartment as an intermediate step. To learn more about the involvement of endosomes in the recycling of synaptic and secretory vesicles we studied in vitro fusion of early endosomes derived from pheochromocytoma (PC12) cells. Fusion was not affected by cleavage of the SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor) proteins synaptobrevin and syntaxin 1 that operate at the exocytotic limb of the pathway. Furthermore, fusion was inhibited by the fast Ca2+ chelator 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetra-acetic acid but not by the slow Ca2+ chelator EGTA. Endosome fusion was restored by the addition of Ca2+ with an optimum at a free Ca2+ concentration of 0.3 × 10−6 M. Other divalent cations did not substitute for Ca2+. A membrane-permeant EGTA derivative caused inhibition of fusion, which was reversed by addition of Ca2+. We conclude that the fusion of early endosomes participating in the recycling of synaptic and neurosecretory vesicles is mediated by a set of SNAREs distinct from those involved in exocytosis and requires the local release of Ca2+ from the endosomal interior.
INTRODUCTION
Neurotransmitter release in the nerve terminal is mediated by exocytosis of synaptic vesicles. After exocytosis, the vesicle membrane is retrieved by endocytosis and used for the reformation of fusion-competent synaptic vesicles. In recent years, the pathways involved in the vesicle recycling have received considerable attention. Both morphological (Heuser and Reese, 1973; Shupliakov et al., 1997) and genetic evidence (Koenig and Ikeda, 1989; González-Gaitán and Jäckle, 1997) demonstrate that clathrin-coated vesicles are essential intermediates in vesicle recycling, although direct, i.e., non–clathrin-dependent, retrieval mechanisms may coexist in the synapse (Fesce et al. 1996).
Currently, it is still debated to which extent endosomal intermediates are involved in synaptic vesicle recycling. Originally, it was assumed that after pinching off the plasma membrane, clathrin-coated vesicles decoat and subsequently fuse with early endosomes. Synaptic vesicles then regenerate by budding from the endosome. This view was mostly based on analogy to clathrin-mediated recycling pathways in non-neuronal cells (Goldstein et al., 1985; Kelly, 1993). Membrane cisternae larger than synaptic vesicles are visible in nerve terminals, at least after intense stimulation, which may correspond to endosomes (Heuser and Reese, 1973). In addition, rab5, a resident of early endosomes (Chavrier et al., 1990), is highly enriched in synapses, suggesting that early endosomes play a prominent role in the synaptic vesicle pathway (Fischer von Mollard et al., 1994). However, recent data suggest that synaptic vesicles may recycle directly without intermediate fusion and budding steps. When vesicles undergoing endocytosis are labeled with the styryl dye FM 1-43, it was found that the amount of dye a vesicle releases upon subsequent exocytosis equals the amount taken up by endocytosis (Murthy and Stevens, 1998), ruling out communication with intermediate endosomal compartments. Additional evidence for direct recycling was obtained when the biogenesis of synaptic vesicles was studied in PC12 cells. Here, vesicles are generated in parallel by two clearly distinguishable pathways. One of them presumably involves direct retrieval from the plasma membrane (Shi et al., 1998). In the second pathway synaptic vesicles bud off from endosomal precursors in a step that is Arf and AP-3 dependent and inhibited by brefeldin A (Faúndez et al., 1997, 1998; Lichtenstein et al., 1998). Thus it appears that endosomes may be bypassed during recycling of synaptic vesicles. Nevertheless, the available data also suggest that synaptic vesicles pass through endosomes at least occasionally during repetitive recycling. Clearly, vesicle constituents are subject to endocytic sorting, an event that requires endosomes as functional sorting compartments.
Synaptic vesicles that passage through endosomal intermediates undergo two distinct budding and fusion steps in each cycle. Exocytotic fusion is well characterized. It is highly regulated by intracellular Ca2+-concentrations and is mediated by a set of conserved membrane proteins including synaptobrevin, syntaxin, and SNAP-25, commonly also referred to as SNAREs (soluble N-ethylmaleimide-sensitive factor [NSF] attachment protein receptors). Botulinum and tetanus toxins irreversibly block exocytosis by means of selectively proteolyzing these exocytotic SNAREs (Niemann et al., 1994; Montecucco and Schiavo, 1995). The neuronal SNARE proteins are representatives of a large protein superfamily that appears to be involved in virtually every intracellular fusion reaction. Although still debated, it is currently thought that SNARE assembly directly mediates membrane fusion (Jahn and Hanson, 1998).
The mechanisms of endosome fusion in this pathway are less well understood. However, endosome fusion has been investigated in some detail in non-neuronal cells, mostly because of the availability of convenient cell-free fusion assays (Braell, 1987; Gruenberg and Howell, 1987; Diaz et al., 1989). Both fusion of incoming endocytotic compartments with early endosomes as well as of endosomal vesicles with each other has been described (Mayorga et al., 1988; Diaz et al., 1989; Woodman and Warren, 1991). However, early endosomes cannot fuse with carrier vesicles shuttling between early and late endosomes, or with late endosomes indicating that specific proteins are required for each fusion event (Braell, 1987; Gruenberg and Howell, 1987; Gruenberg et al., 1989). Fusion is sensitive to ionic environment (Diaz et al., 1993) and requires ATP and both soluble and membrane-bound proteins (Diaz et al., 1989). The small GTPase rab5 plays an essential role in endosome fusion (Gorvel et al., 1991). Several putative effector proteins have been described for rab5, including rabaptin-5 (Stenmark et al., 1995), rabex-5 (Horiuchi et al., 1997), and EEA1 (Simonsen et al., 1998), which may operate in conjunction with rab5. Studies on the fusion of vacuole precursors in yeast have suggested that rab proteins and their effectors are required for membrane attachment but do not participate in the fusion reaction itself (Ungermann et al., 1998).
Currently, it is unknown which SNARE proteins mediate endosome fusion. During synaptic vesicle recycling, considerable amounts of all three synaptic SNAREs are endocytosed (Walch-Solimena et al., 1995). These proteins are functionally “active” because they form SNARE complexes in the membrane, which can be reversibly disassembled by the ATPase NSF (Otto et al., 1997). Recent evidence has shown that there is little specificity in SNARE pairing, because even only distantly related members of the SNARE family can replace a given SNARE in a SNARE complex (Fasshauer et al., 1999; Yang et al., 1999). In addition, it was reported that fusion of endosomes derived from fibroblasts (BHK-21 cells) is insensitive to pretreatment with clostridial neurotoxins (Link et al., 1993), whereas fusion of aquaporin-containing endosomes derived from kidney papillae is inhibited by tetanus toxin (TeNT) (Jo et al., 1995). Thus it is conceivable that exocytotic SNAREs also function in endosome fusion. Furthermore, calmodulin has been invoked in intracellular fusion events, including endosome fusion (Colombo et al., 1997), raising the possibility that endosome fusion, like exocytosis, is dependent on a rise of intracellular calcium.
In the present study, we have investigated the fusion of early endosomes involved in synaptic vesicle recycling using the neuroendocrine cell line PC12 as a model. PC12 cells possess two types of secretory vesicles: secretory granules (large dense-core vesicles) containing dopamine and protein and small synaptic vesicles containing acetylcholine (Bauerfeind et al., 1993). Although their exocytosis is presumably differentially regulated, they share a common pool of endosomes during recycling (Bauerfeind et al., 1995). Our results show that endosome fusion does not involve exocytotic SNAREs and requires local release of calcium from intraorganellar stores.
MATERIALS AND METHODS
Antibodies
The following antibodies were described previously: synaptobrevin (monoclonal antibody Cl 69.1) (Edelmann et al., 1995), synaptophysin (monoclonal antibody C 7.2) (Jahn et al., 1985), SNAP-25 (Cl 71.1) (Bruns et al., 1997), and cellubrevin (rabbit antiserum) (Annaert et al., 1997). For NSF, mouse monoclonal antibodies were generated using recombinant NSF as antigen. These antibodies, which will be described in detail elsewhere (Rammner, Otto, and Jahn, manuscript in preparation), recognized a single band in immunoblots corresponding to the position of NSF. The following antibodies were kind gifts: syntaxin (monoclonal antibody HPC-1; kindly provided by Dr. C. Barnstable, Yale University, New Haven, CT) and sec61α (rabbit serum; kindly provided by Dr. E. Hartmann, Göttingen University, Göttingen).
Fluid Phase Internalization
When BHK-21 cells were used, the experiments were carried out essentially as described previously (Gruenberg et al., 1989, 1991; Link et al., 1993). For PC12 cells (Greene and Tischler, 1976) the protocol was slightly modified. Briefly, PC12 cells were grown to confluency on collagen-coated culture dishes in RPMI 1640 medium supplemented with 5% heat-inactivated fetal calf serum and 10% heat-inactivated horse serum (Life Technologies, Gaithersburg, MD), 20 mM HEPES, pH 7.4, 4 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin at 37°C in 7.5% CO2. Cells were harvested by pipetting with ice-cold saline PBS and washed repeatedly. Cells were washed once in prewarmed internalization medium (OptiMEM; Life Technologies) supplemented with 10 mM d-glucose). Ten confluent plates of a diameter of 150 mm resulted in a cell pellet of ∼1.5 ml vol. The cell pellet was resuspended in 4 vol (vol/vol) of internalization medium that contained either 3.2 mg/ml avidin (Pierce, Rockford, IL) or 1.7 mg/ml biotinylated HRP (prepared according to the method of Gruenberg et al., 1991) for the acceptor and donor compartments, respectively, and incubated for 5 min at 37°C. The cells were rapidly diluted with 3–4 vol of ice-cold PBS containing 5 mg/ml BSA and washed four times at 4°C.
Preparation of Subcellular Fractions
All steps of the preparation were carried out at 4°C or on ice. After fluid phase internalization (see above) the cell pellet was resuspended in 4 vol (vol/vol) of homogenization buffer (250 mM sucrose, 3 mM imidazole-HCl, pH 7.4, and protease inhibitors: 10 μg/ml trypsin inhibitor, 0.7 μg/ml pepstatin, and 0.1 mM PMSF) and homogenized by 20 passages through a stainless steel ball homogenizer with a clearance of 0.0005 inches and eight passages with a clearance of 0.0009 inches for PC12 and BHK-21 cells, respectively. The homogenates were centrifuged for 15 min at 1800 × g. The resulting postnuclear supernatants were divided into aliquots, snap frozen in liquid N2, and stored at −70°C until use. Dilutions of these samples were used as sources for either the acceptor compartment (avidin) or the donor compartment (biotinylated HRP) in the fusion assay. HRP uptake into organelles was determined by measuring the amount of HRP recovered in the membrane fraction that was obtained by centrifugation of the postnuclear supernatant at 45,000 × g for 30 min using a TLA-100.3 rotor (Beckman Instruments, Palo Alto, CA). In the membrane fraction an average of 50–200 ng HRP/mg protein of the postnuclear supernatants was internalized.
For the preparation of cytosol, BHK-21 or PC12 cells were resuspended in 3 vol of homogenization buffer and homogenized in a stainless steel ball homogenizer with 10 passages (clearance, 0.0009 inches) or 20 passages (clearance, 0.0005 inches), respectively. A postnuclear supernatant obtained as described above was centrifuged at 14,000 × g for 25 min. The resulting low-speed supernatant was centrifuged at 185,000 × g for 1 h in a TLA-100.3 rotor. The high-speed supernatant was divided into 500-μl aliquots, snap frozen in liquid N2, and stored at −70°C until use. For the preparation of cytosol from rat brain, brains were homogenized in homogenization buffer (1 ml/g tissue) using a glass-Teflon homogenizer. The homogenate was centrifuged at 3500 × g for 20 min. A high-speed supernatant was obtained as described above.
Cell-free Fusion Assay
The assay for in vitro fusion of early endosomes of PC12 and BHK-21 cells and PC12–BHK-21 mixed cells was performed as previously described (Gruenberg et al., 1989; Gorvel et al., 1991; Link et al., 1993) with minor modifications.
Reaction mixtures (200 μl in total) were assembled on ice, containing, as final concentrations, postnuclear supernatants (4 mg protein/ml), cytosol (2.5 mg/ml; if not indicated otherwise, cytosol from rat brain was used), 11.25 mM HEPES, pH 7.0, 1.35 mM magnesium acetate, 0.18 mM dithiothreitol, 45 mM potassium acetate, 0.05 mg/ml biotinylated insulin as quencher, and as ATP-regenerating system 1.6 mM ATP, 13 mM creatine phosphate, and 0.066 mg creatine phosphokinase (800 U/mg; Boehringer Mannheim, Mannheim, Germany). Quantification of the enzymatic activity of biotinylated HRP was performed as described (Al-Kassai and Mostratos, 1983). Fusion efficiency was calculated by relating the amount of biotinylated HRP recovered in the immunoprecipitated complex to the amount of HRP recovered in the membrane fraction of the postnuclear supernatant. ATP-dependent fusion efficiency of PC12 cell endosomes, measured under standard conditions, ranged between 25 and 30%.
For treatment with N-ethylmaleimide (NEM), both postnuclear supernatants and cytosol were preincubated separately with 1 mM NEM at 37°C for 30 and 10 min, respectively. For the experiment shown in Figure 1, NEM pretreatment was performed for only 15 min on ice, followed by the addition of 2 mM DTT and another 10-min incubation on ice. For incubation with light chains (L chains) of TeNT or of botulinum neurotoxin C1 (BoNT/C1), the postnuclear supernatants were preincubated separately at 37°C for 30 min in the presence of active or heat-inactivated toxin L chains. An aliquot was removed before the fusion reaction to check for substrate cleavage. Stock solutions for 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetra-acetic acid (BAPTA; Molecular Probes, Eugene, OR), EGTA, and EGTA-acetoxymethyl ester (AM) (Calbiochem, La Jolla, CA; 200 mM) were carefully adjusted to neutral pH. For treatment with EGTA-AM postnuclear supernatants were preincubated for 30 min on ice and 30 min at room temperature with EGTA-AM (dissolved in DMSO). Controls for the effect of the solvent alone in our endosome fusion assay were also performed.
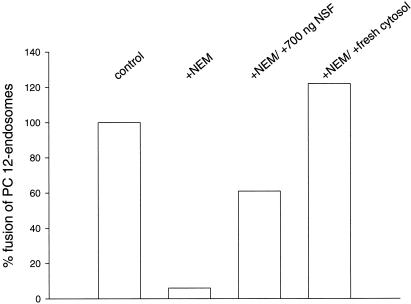
Figure 1.
Fusion of PC12 cell-derived endosomes in vitro is inhibited by NEM and restored by subsequent addition of purified NSF or untreated cytosol. The data are derived from a representative experiment.
Immunoisolation
Monoclonal antibodies C 7.2 were covalently coupled to the reactive surface of Eupergit C1Z methacrylate microbeads (1 μm mean diameter; Roehm Pharma, Darmstadt, Germany) as described (Burger et al., 1989). Antibody-containing immunobeads are referred to as synaptophysin beads. Fusion assays were performed as indicated above using PC12 postnuclear supernatants (1 mg protein/ml) and cytosol from rat brain (2.5 mg protein/ml). After completing the fusion reaction, 6.25 μl of synaptophysin beads and control beads, respectively, were added, followed by 30 min of incubation on a rotator at 4°C. The sample was then diluted in 3 vol homogenization buffer, layered on top of a sucrose cushion (0.5 ml, 0.8 M), and centrifuged for 5 min at 4600 × g in a microfuge. The supernatants were subjected to a high-speed centrifugation step for 30 min at 200,000 × g at 4°C using a Beckman TLA-120.2 rotor to sediment nonbound membranes. The bead pellets were resuspended in 1 ml fusion assay buffer and centrifuged for 5 min at 1800 × g. An aliquot of each sample was then solubilized in detergent and the HRP–avidin complex was immunoprecipitated and quantitated as described to determine fusion activity. A second aliquot was analyzed by SDS-PAGE and immunoblotting.
Other Methods
Plasmids encoding His6-TeNT L chain and His6-BoNT/C1 L chain were kindly provided by Dr. Heiner Niemann (Medizinische Hochschule, Hannover, Germany). Fusion proteins were purified on Ni2+-Sepharose columns according to the manufacturer’s protocol (PROBond; Invitrogen, San Diego, CA). Purity was examined by SDS-PAGE and staining with Coomassie blue. The proteins were dialyzed against HEPES-buffered saline (20 mM HEPES, pH 7.4, 150 mM NaCl, and 1 mM dithiothreitol) and stored at −70°C until use. Recombinant NSF was expressed in bacteria and purified as described (Hanson et al., 1997).
SDS-PAGE and immunoblotting were performed according to standard procedures (Laemmli, 1970). For detection, we used either the enhanced chemiluminescence kit of Amersham (Arlington Heights, IL; HRP-conjugated antibodies) or a colorimetric procedure involving the generation of formazan precipitates (Sambrook et al., 1989).
RESULTS
Endosome Fusion Involves Endosomes of the Synaptic Vesicle Recycling Pathway
In the first series of experiments we investigated whether endosome fusion as measured by our assay includes endosomes involved in synaptic or secretory vesicle recycling. Fusion was monitored using an established procedure, which is based on content mixing of two different endosomal populations derived from preloaded cells. PC12 cells were loaded for 5 min either with biotinylated horseradish peroxidase (HRP, donor compartment) or with avidin (acceptor compartment). Endosome-containing cell-free extracts from both cell populations were mixed in the presence of cytosol and ATP. When fusion occurs, a tight complex forms between avidin and biotinylated HRP, which is isolated by immunoprecipitation of avidin and quantitated by measuring HRP activity. To prevent formation of biotinylated HRP–avidin complexes derived from disrupted endosomes, biotinylated insulin was added as quencher. In accordance with endosome fusion reported from other cell lines, fusion was dependent on ATP (also see Figure 6A). Similarly, fusion is prevented by pretreatment of postnuclear supernatants and cytosol with NEM. This inhibition is reversed by the subsequent addition of fresh cytosol (Figure 1). Purified NSF partially substitutes for cytosol in this experiment (Figure 1), in agreement with previous reports from other cell lines (e.g., Diaz et al., 1989).
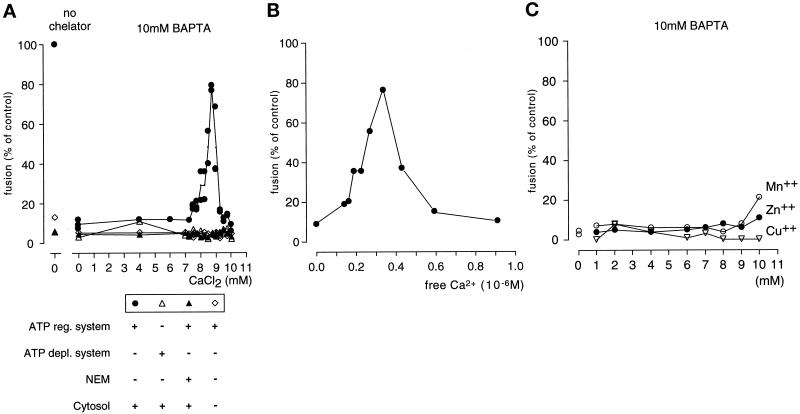
Figure 6.
Fusion of early endosomes from PC12 cells is dependent on Ca2+. (A) Endosome fusion inhibited by BAPTA is restored by the addition of CaCl2. No fusion is observed at any Ca2+ concentration when cytosol is omitted, ATP is depleted, or the extracts are pretreated with NEM. (B) Calculation of the free Ca2+ concentration, based on the data shown in the left panel. Fusion was optimal at a free Ca2+ concentration of 0.3 μM. For calculations the program of Föhr et al. (1993) was used. (C) Mn2+, Zn2+, and Cu2+ ions are unable to substitute for Ca2+ in promoting fusion of early endosomes. The results are representative of three (A and B) and two (C) independent experiments.
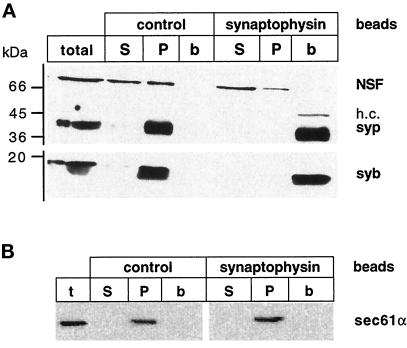
Synaptophysin is specifically localized to synaptic vesicles and, to a lesser extent, to secretory vesicles in PC12 cells and is widely used as a recycling marker in studies addressing vesicle recycling (Linstedt and Kelly, 1991). We therefore used immobilized monoclonal antibodies specific for synaptophysin to immunoisolate all synaptophysin-containing compartments at the end of the fusion reaction and then examined to which extent the fused compartments were bound to the immunobeads. For immunoisolation we used Eupergit C1Z beads, which were shown to yield exceptionally clean fractions with negligible nonspecific binding (Cameron et al., 1991). Immunoisolation of synaptophysin-containing membranes from the fusion assay mix resulted in quantitative binding of all synaptophysin-containing membranes, with virtually no synaptophysin remaining unbound (Figure 2A). No binding was observed when control beads containing no antibody were used. To further control for nonspecific binding of membranes to the immunobeads, we used sec61α, a membrane constituent of the protein import complex from the endoplasmic reticulum, as a marker. As shown in Figure 2B, no sec61α was found in the bead fraction, confirming that the isolation procedure is highly specific.
Figure 2.
Binding of fused early endosomes to synaptophysin immunobeads. (A) Aliquots of membranes isolated with synaptophysin beads and control beads (b) as well as their corresponding supernatants (S) and high-speed membrane pellets (P) were analyzed by SDS-PAGE and immunoblotting for synaptic vesicle proteins. In the first lane an aliquot of the total fusion reaction before immunisolation was loaded. Note that in addition to endosomes, all synaptophysin-containing membranes are indiscriminately bound to the beads including synaptic-like microvesicles (Cameron et al., 1991). h.c., heavy chain of the antibody C 7.2; syp, synaptophysin; syb, synaptobrevin. (B) Specificity of the immunobeads. The same fractions were analyzed for the distribution of the endoplasmic reticulum-resident sec61α.
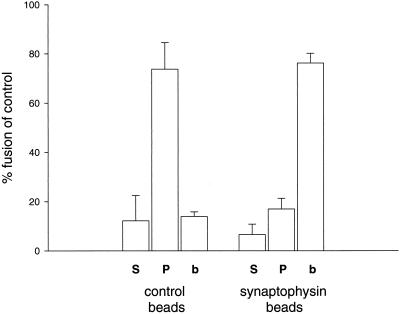
We then examined the distribution of the fused endosomes in the various fractions. As shown in Figure 3, the vast majority of reaction product was recovered in the immunobead fraction. When inactivated beads were used, most of the reaction product remained in the supernatant, again demonstrating the specificity of the immunoisolation procedure. When membranes present in this supernatant were sedimented by centrifugation, most of the reaction product was recovered in the pellet. This proves that endosomal lysis was negligible in the experiment, because lysis would release the reaction product into the soluble fraction. We conclude that early endosome fusion as monitored by our assay predominantly involves endosomes participating in the trafficking pathway of secretory and synaptic vesicles.
Figure 3.
Fused early endosomes were bound to synaptophysin immunobeads. The amount of fusion product in the different fractions, supernatant (S), membrane pellet (P), and bead pellet (b), was monitored. Fusion activity under standard conditions is defined as 100%. The data are represented as means with SD from four independent experiments.
SNAREs Mediating Exocytosis of Synaptic Vesicles Are Not Involved in Endosome Fusion
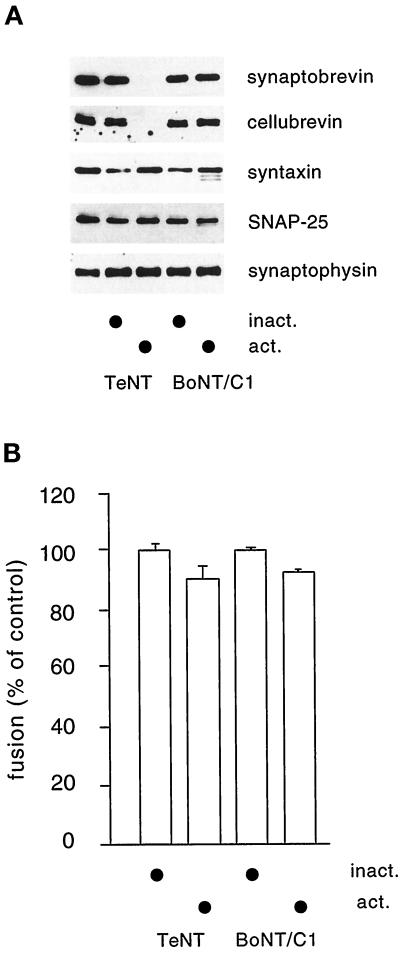
We next studied whether the SNARE proteins involved in calcium-dependent exocytosis of synaptic vesicles and secretory granules play a role in endosome fusion in PC12 cells. As outlined in INTRODUCTION, the SNARE proteins synaptobrevin 2, syntaxin 1, and SNAP-25 are essential for exocytotic membrane fusion and are present on endocytic compartments. To inactivate exocytotic SNAREs, cell-free extracts were incubated with the L chains of TeNT (specific for synaptobrevin), BoNT C1 (specific for syntaxin 1), and BoNT A (specific for SNAP-25; our unpublished results) before the fusion assay. Incubation with TeNT L chain resulted in virtually complete cleavage of synaptobrevin and its close relative cellubrevin (Figure 4A). Cleavage of syntaxin by BoNT C1 L chain was less effective (in agreement with earlier results; Blasi et al., 1993), but cleavage products were clearly detectable (Figure 4A). When endosome fusion was monitored, no significant change in fusion activity was observed under any of the conditions examined (Figure 4B). The fusion reaction was still ongoing at the time the incubation was terminated (our unpublished results; also see Link et al., 1993), suggesting that not only the extent but also the rate of fusion was unaffected by toxin treatment. We conclude that fusion of early endosomes in PC12 cells involves SNAREs different from syntaxin 1 and synaptobrevin/cellubrevin.
Figure 4.
Fusion of early endosomes from PC12 cells is not inhibited by preincubation of the cell-free extract with TeNT-L chain and BoNT/C1-L chain. (A) Aliquots of the toxin-treated extracts were analyzed by SDS-PAGE and immunoblotting for substrate breakdown. In the first lane untreated cell-free extract was loaded. Note that the syntaxin signal is variable; however, partial breakdown is evident by the appearance of bands of increased mobility reflecting cleavage products. No higher degree of breakdown was observed when the BoNT/C1 L chain concentration was increased 50-fold (our unpublished results). (B) Cell-free extracts were incubated for 30 min before the fusion assay with 10 nM TeNT and BoNT/C1, respectively, using either active (act.) or heat-inactivated (inact.) recombinant toxin L chains. The data were derived from five independent experiments.
Ca2+ Dependence of Early Endosome Fusion in PC12 Cells
Next we analyzed whether the fusion of early endosomes is dependent on the presence of calcium ions for two reasons. First, we wanted to determine whether endosome fusion involved in the recycling of membranes exocytosing in a Ca2+-dependent manner is also dependent on Ca2+, thus resembling exocytotic fusion. Second, evidence has recently accumulated showing that Ca2+- and/or calcium-binding proteins are generally required for intracellular fusion events. For instance, activation of calmodulin appears to be involved in the fusion of endosomes and yeast vacuole precursors (Colombo et al., 1997; Peters and Mayer, 1998). In the latter case it has been found that the calmodulin-dependent step operates after SNARE assembly. It was suggested, that this step is an essential component of the overall fusion reaction. Furthermore, in vitro fusion of nuclear envelope membranes depends on the local release of Ca2+ ions from an unknown vesicular store that is probably mediated by inositol 1,4,5-triphosphate (IP3) receptors (Sullivan et al., 1993). The Ca2+-binding protein that is acted on by Ca2+ is not known in this case. The experiments described here should therefore clarify whether 1) fusion of early endosomes from PC12 cells is dependent on calcium; 2) calcium required for fusion is derived from a local pool; and 3) Ca2+ dependence is a general feature of endosome fusion irrespective of the cell type and recycling pathway.
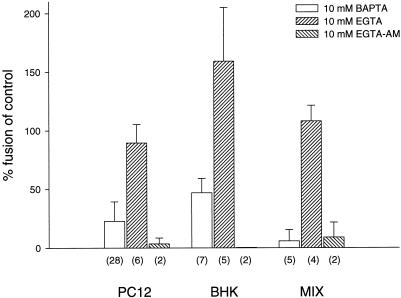
First, we used the chelator BAPTA to reduce the Ca2+ concentration during the fusion reaction. When the assay was performed in the presence of 10 mM BAPTA, fusion was largely inhibited (Figure 5). In contrast, no inhibition was observed when EGTA was added at equal concentrations (Figure 5). To test whether the sensitivity to BAPTA is common to all endosome fusion reactions, we compared fusion of PC12 cell-derived endosomes with that of fibroblast-derived endosomes (BHK-21 cells). As shown in Figure 5, fusion of BHK-21 cell-derived endosomes was also inhibited, but to a lesser degree. The result was independent from the source of cytosol used to support the fusion reaction, including rat brain, PC12 cells, and BHK-21 cells (our unpublished results), and excluding that the differential sensitivity to chelators is due to a tissue-specific cytosolic factor. Similar results were obtained in mixed fusion reactions in which donor and acceptor populations of endosomes were derived from PC12 and BHK-21 cells, respectively (Figure 5, right).
Figure 5.
In vitro fusion of early endosomes is inhibited by BAPTA and EGTA-AM but not by EGTA. Fusion was performed under standard conditions (see MATERIALS AND METHODS for details). The numbers in parentheses give the numbers of independent experiments. Error bars represent SD.
Because both BAPTA and EGTA have very similar affinities for various divalent cations at neutral pH, the strikingly different effects of these chelators on endosome fusion cannot be explained by cation preference. However, BAPTA has >100-fold higher ion association and dissociation rates than EGTA (Tsien, 1980; Fabiato, 1985; Adler et al., 1991). This difference allows for the discrimination of processes that are dependent on fast and local changes of Ca2+ concentrations. For example, exocytosis in neurons is sensitive to intracellularly applied BAPTA but largely resistant to EGTA (Adler et al., 1991). Thus, our data suggest that fusion of early endosomes is mediated by a rapid and local release of Ca2+ from an intracellular store that is close to, probably identical to, the endosomes participating in the fusion reaction. To test this hypothesis, we examined the effects of a membrane-permeable derivative of EGTA on endosome fusion. Preincubation with such an analogue is expected to chelate Ca2+ in vesicular stores that are inaccessible to BAPTA. As shown in Figure 5, preincubation of the endosome-containing extracts with EGTA-AM inhibited fusion by >80% irrespective of whether PC12-derived or BHK-21-derived endosomes were used. We conclude that Ca2+ released locally from an intracellular store plays an important regulatory role in triggering endosome fusion.
Both EGTA and BAPTA preferentially chelate Ca2+ ions but they are not entirely cation selective. Using PC12 cell endosomes, we therefore tested whether fusion was restored by Ca2+ and/or other divalent cations. When increasing concentrations of CaCl2 were added in the presence of 10 mM BAPTA, fusion returned to almost normal levels (85% of control; Figure 6A). Further increase of CaCl2 reduced fusion. The free Ca2+ concentration yielding maximal fusion was calculated to be 0.3 μM (Figure 6B). Under all conditions, fusion was strictly dependent on the presence of ATP and cytosol and was sensitive to inhibition by NEM (Figure 6A). None of the other divalent cations tested was able to substitute for Ca2+ (Figure 6C). Similarly, the block caused by EGTA-AM was reversed by increasing the free Ca2+ concentration (our unpublished results). Together, these results demonstrate that fusion of early endosomes is dependent on the local release of Ca2+ and requires Ca2+ concentrations that are severalfold higher than the resting levels normally found in cells.
DISCUSSION
In this study we have demonstrated that in the neuroendocrine cell line PC12 fusion of early endosomes predominantly involves recycling membranes of the regulated secretory pathway. Furthermore, the data document that the SNAREs mediating endosome fusion are most probably different from the SNAREs involved in exocytosis. PC12–endosome fusion is dependent on the local release of Ca2+ from intraorganellar stores, a feature that appears to be shared by non-neuronal cells.
In the original version of the SNARE hypothesis, it was postulated that each intracellular fusion step is mediated by a unique set of SNAREs. Recently, however, it has become clear that some SNAREs operate in multiple trafficking steps, for example, the yeast Q-SNAREs Sed5p and Vti1p (Götte and Fischer von Mollard, 1998). In particular, there is currently no direct evidence supporting the idea that in a cyclic trafficking pathway (e.g., vesicular traffic between the endoplasmic reticulum and the Golgi apparatus) the anterograde and retrograde fusion steps, respectively, are mediated by different sets of SNAREs. Furthermore, synaptobrevin, like Sed5p, forms complexes with multiple syntaxins in vitro, and a similar promiscuity was observed for syntaxin isoforms. These findings suggest that these proteins may participate in multiple SNARE complexes. However, our data clearly show that the fusion of endocytosed membranes of secretory vesicles involves SNAREs different from those operating during exocytosis of the vesicles. The SNAREs mediating fusion are not yet known, although some SNAREs (endobrevin/VAMP8, syntaxin 7, and syntaxin 12/13) have recently been localized to early endosomes (Advani et al., 1998; Prekeris et al., 1998; Tang et al., 1998; Wong et al., 1998a,b). Intriguingly, we have previously reported that clathrin-coated vesicles involved in synaptic vesicle recycling not only contain the R-SNARE synaptobrevin but also the Q-SNAREs syntaxin and SNAP-25 (i.e., a full set of exocytotic SNAREs), and furthermore, that these proteins form NSF-sensitive ternary complexes (cis-complexes) in these membranes (Otto et al., 1997). Thus, early endosomes may contain two or more sets of SNAREs, which recycle together with the other vesicle constituents. Consequently, mechanisms need to exist that define which of the resident SNARE proteins in the membrane is used for a given fusion step.
One of the most striking observations is that endosome fusion was strongly inhibited by the fast Ca2+ chelator BAPTA but was not affected by the slower Ca2+ chelator EGTA despite identical affinities of both chelators for Ca2+. Insensitivity to EGTA is in agreement with an earlier study (Mayorga et al., 1994). In contrast, the membrane-permeant analogue of EGTA, EGTA-AM, inhibited the reaction. These results allow us to conclude 1) that the Ca2+ pool promoting fusion is derived from a local pool, probably only a few nanometers away from the Ca2+ receptor; and 2) that this local pool resides in the interior of a vesicular compartment, most likely the endosomes themselves. Similar observations were reported previously from the fusion of nuclear membranes (Sullivan et al., 1993) and, more recently, from the fusion of vacuolar precursor membranes derived from yeast (Peters and Mayer, 1998). In these cases, local release of Ca2+ was needed for fusion, raising the possibility that Ca2+ release from intraorganellar stores is a general requirement for intracellular fusion reactions.
Which is the protein Ca2+ is acting on in these fusion reactions? In the fusion of yeast vacuoles, calmodulin has emerged as a strong candidate, suggesting that a calmodulin-binding protein operates in the control of membrane fusion. Using stage-specific inhibitors of the fusion reaction, it was demonstrated that calmodulin exerts its effect at a late step in the fusion reaction, probably after assembly of the SNARE complex. Furthermore, calmodulin antagonists were previously shown to block endosome fusion in a macrophage cell line, and this inhibition was reversed by the addition of purified calmodulin (Colombo et al., 1997). A partial inhibition of endosome fusion by calmodulin antagonists was also observed in our experiments (our unpublished observations). Together, these observations suggest that calmodulin may be involved in mediating the Ca2+ effects described here. The targets of calmodulin remain to be established. The protein EEA1, which possesses a calmodulin binding domain, has recently been implicated as an effector of Rab5. Thus it is possible that at least parts of the results obtained here may be attributed to a regulation of EEA1. Another candidate Ca2+ target is the Ca2+-binding protein annexin II, which was identified as a major component of early endosomes (Emans et al., 1993) and which appears to play an as yet undefined role in endosome fusion (Mayorga et al., 1994).
How is Ca2+ getting into the early endosome? Clearly, one of the major Ca2+ sources for endocytotic organelles is contributed by the extracellular fluid, which contains Ca2+ concentrations in the millimolar range. It remains to be established whether in addition an active refilling mechanism operates, e.g., by means of a Ca2+-ATPase. Because early endosomes are transient compartments, active uptake may not be required to maintain a high intraendosomal Ca2+ concentration. This view is supported by the fact that thapsigargin, an inhibitor of the members of the sarcoplasmic and endoplasmic reticulum Ca2+-ATPases, did not affect Ca2+-dependent fusion of early endosomes even when added at supramaximal concentrations (our unpublished results). Alternatively, Ca2+ sequestration may occur through a thapsigargin-insensitive intracellular calcium pump distinct from the classical sarco(endo)plasmic reticulum Ca2+-ATPases (Waldron et al., 1995). Recently, an intracellular Ca2+ pool that is insensitive to thapsigargin and IP3 has been observed in mammalian cell lines, including PC12 cells (Pizzo et al., 1997), which may be related to the pool described here.
In summary, the endosome fusion reaction in PC12 cells has some intriguing similarities with neuronal exocytosis but also important differences. In both cases, local release of Ca2+ drives membrane fusion. During exocytosis, Ca2+ release is triggered by depolarization, which leads to the opening of voltage-gated Ca2+ channels clustering at the release sites. In endosome fusion, the events triggering Ca2+ release are unknown. It is possible that recognition and/or docking of the fusion partners generates a signal that activates endosomal Ca2+ channels, for instance, IP3 or polyphosphoinositides. Alternatively, it is possible that putative endosomal Ca2+ channels fire spontaneously, creating local Ca2+ gradients that are only effective when the participating membranes are close to each other. The molecular mechanisms involved in Ca2+-mediated control of endosome fusion remain to be established.
ACKNOWLEDGMENTS
We are greatly indebted to Dr. H. Niemann for the gift of plasmids, Drs. E. Hartmann and C. Barnstable for the gift of antibodies, and M. Margittai (Max-Planck-Institute for Biophysical Chemistry, Göttingen, Germany) for providing us with purified NSF. Furthermore, we thank one of the reviewers for suggesting important control experiments we had omitted in an earlier version. U.K. was the recipient of a fellowship from the Boehringer-Ingelheim fonds. W.A. was supported by a D. Collen Fellowship and a Philips Fellowship from the Belgian American Educational Foundation, a Fulbright-Hays Award, and a North American Treaty Organization grant.
REFERENCES
- Adler EM, Augustine GJ, Duffy SN, Charlton MP. Alien intracellular calcium chelators attenuate neurotransmitter release at the squid giant synapse. J Neurosci. 1991;11:1496–1507. doi: 10.1523/JNEUROSCI.11-06-01496.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Advani RJ, Bae H-R, Bock JB, Chao DS, Doung Y-C, Prekeris R, Yoo J-S, Scheller RH. Seven novel mammalian SNARE proteins loclize to distinct membrane compartments. J Biol Chem. 1998;273:10317–10324. doi: 10.1074/jbc.273.17.10317. [DOI] [PubMed] [Google Scholar]
- Al-Kassai E, Mostratos A. Assessment of substrates for horseradish peroxidase in enzyme immunoassay. J Immunol Methods. 1983;58:127–132. doi: 10.1016/0022-1759(83)90269-7. [DOI] [PubMed] [Google Scholar]
- Annaert WG, Becker B, Kistner U, Reth M, Jahn R. Export of cellubrevin from the endoplasmic reticulum is controlled by BAP31. J Cell Biol. 1997;139:1397–1410. doi: 10.1083/jcb.139.6.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauerfeind R, Jelinek R, Hellwig A, Huttner WB. Neurosecretory vesicles can be hybrids of synaptic vesicles and secretory granules. Proc Natl Acad Sci USA. 1995;92:7342–7346. doi: 10.1073/pnas.92.16.7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauerfeind R, Régnier-Vigouroux A, Flatmark T, Huttner WB. Selective storage of acetylcholine, but not catecholamines, in neuroendocrine synaptic-like microvesicles of early endosomal origin. Neuron. 1993;11:105–121. doi: 10.1016/0896-6273(93)90275-v. [DOI] [PubMed] [Google Scholar]
- Blasi J, Chapman ER, Yamasaki S, Binz T, Niemann H, Jahn R. Botulinum neurotoxin C1 blocks neurotransmitter release by means of cleaving HPC-1/syntaxin. EMBO J. 1993;12:4821–4828. doi: 10.1002/j.1460-2075.1993.tb06171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braell WA. Fusion between endocytic vesicles in a cell-free system. Proc Natl Acad Sci USA. 1987;84:1137–1141. doi: 10.1073/pnas.84.5.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns D, Engers S, Yang C, Ossig R, Jeromin A, Jahn R. Inhibition of transmitter release correlates with the proteolytic activity of tetanus toxin and botulinus toxin A in individual cultured synapses of Hirudo medicinalis. J Neurosci. 1997;17:1898–1910. doi: 10.1523/JNEUROSCI.17-06-01898.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger PM, Mehl E, Cameron PL, Maycox PR, Baumert M, Lottspeich F, De Camilli P, Jahn R. Synaptic vesicles immunoisolated from rat cerebral cortx contain high levels of glutamate. Neuron. 1989;3:715–720. doi: 10.1016/0896-6273(89)90240-7. [DOI] [PubMed] [Google Scholar]
- Cameron PL, Südhof TC, Jahn R, De Camilli P. Colocalization of synaptophysin with transferrin receptors: implications for synaptic vesicle biogenesis. J Cell Biol. 1991;115:151–164. doi: 10.1083/jcb.115.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavrier P, Parton RG, Hauri HP, Simons K, Zerial M. Localization of low molecular weight GTP binding proteins to exocytic and endocytic compartments. Cell. 1990;62:317–329. doi: 10.1016/0092-8674(90)90369-p. [DOI] [PubMed] [Google Scholar]
- Colombo MI, Beron W, Stahl PD. Calmodulin regulates endosome fusion. J Biol Chem. 1997;272:7707–7712. doi: 10.1074/jbc.272.12.7707. [DOI] [PubMed] [Google Scholar]
- Diaz R, Mayorga LS, Colombo MI, Lenhard JM, Stahl PD. In vitro studies of endocytic vesicle fusion. Methods Enzymol. 1993;221:207–222. doi: 10.1016/0076-6879(93)21018-4. [DOI] [PubMed] [Google Scholar]
- Diaz R, Mayorga LS, Weidman PJ, Rothman JE, Stahl PD. Vesicle fusion following receptor-mediated endocytosis requires a protein active in golgi transport. Nature. 1989;339:398–400. doi: 10.1038/339398a0. [DOI] [PubMed] [Google Scholar]
- Edelmann L, Hanson PI, Chapman ER, Jahn R. Synaptobrevin binding to synaptophysin: a potential mechanism for controlling the exocytotic fusion machine. EMBO J. 1995;14:224–231. doi: 10.1002/j.1460-2075.1995.tb06995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emans N, Gorvel J-P, Walter C, Gerke V, Kellner R, Griffiths G, Gruenberg J. Annexin II is a major component of fusogenic endosomal vesicles. J Cell Biol. 1993;120:1357–1369. doi: 10.1083/jcb.120.6.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Time and calcium dependence of activation of calcium-induced release of calcium from the sarcoplasmic reticulum of a skinned canine cardiac Purkinje cell. J Gen Physiol. 1985;85:247–289. doi: 10.1085/jgp.85.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasshauer D, Antonin W, Margittai M, Pabst S, Jahn R. Mixed and noncognate SNARE complexes. J Biol Chem. 1999;274:15440–15446. doi: 10.1074/jbc.274.22.15440. [DOI] [PubMed] [Google Scholar]
- Faúndez V, Horng J-T, Kelly RB. ADP ribosylation factor 1 is required for synaptic vesicle budding in PC12 cells. J Cell Biol. 1997;138:505–515. doi: 10.1083/jcb.138.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faúndez V, Horng J-T, Kelly RB. A function for the AP3 coat complex in synaptic vesicle formation from endosomes. Cell. 1998;93:423–432. doi: 10.1016/s0092-8674(00)81170-8. [DOI] [PubMed] [Google Scholar]
- Fesce R, Valtorta F, Meldolesi J. The membrane fusion machine and neurotransmitter release. Neurochem Int. 1996;28:15–21. doi: 10.1016/0197-0186(95)00059-h. [DOI] [PubMed] [Google Scholar]
- Fischer von Mollard G, Stahl B, Walch-Solimena C, Takei K, Daniels L, Khoklatchev A, De Camilli P, Südhof TC, Jahn R. Localization of rab5 to synaptic vesicles identifies endosomal intermediate in synaptic vesicle recycling pathway. Eur J Cell Biol. 1994;65:319–326. [PubMed] [Google Scholar]
- Föhr KJ, Warchol W, Gratzl MC. Calculation and control of free divalent cations in solutions used for membrane fusion studies. Methods Enzymol. 1993;221:149–153. doi: 10.1016/0076-6879(93)21014-y. [DOI] [PubMed] [Google Scholar]
- Götte M, Fischer von Mollard G. A new beat for the SNARE drum. Trends Cell Biol. 1998;8:215–218. doi: 10.1016/s0962-8924(98)01272-0. [DOI] [PubMed] [Google Scholar]
- Goldstein JL, Brown MS, Anderson RG, Russell DW, Schneider WJ. Receptor-mediated endocytosis: concepts emerging from the LDL-receptor system. Annu Rev Cell Biol. 1985;1:1–39. doi: 10.1146/annurev.cb.01.110185.000245. [DOI] [PubMed] [Google Scholar]
- González-Gaitán M, Jäckle H. Role of drosophila α-adaptin in presynaptic vesicle recycling. Cell. 1997;88:767–776. doi: 10.1016/s0092-8674(00)81923-6. [DOI] [PubMed] [Google Scholar]
- Gorvel J-P, Chavrier P, Zerial M, Gruenberg J. Rab5 controls early endosome fusion. in-vitro. Cell. 1991;64:915–925. doi: 10.1016/0092-8674(91)90316-q. [DOI] [PubMed] [Google Scholar]
- Greene LA, Tischler AS. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci USA. 1976;73:2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenberg JE, Gorvel J-P, Emans N, Walter C. Subcellular fractionation of endosomes and cell-free analysis of endosomal vesicle fusion. In: Gruenberg JE, Hoflack B, Wandinger-Ness A, editors. Course Manual of the EMBO Practical Course: Subcellular Fractionation of Transport Vesicles. Heidelberg: European Molecular Biology Laboratory; 1991. pp. 1–36. [Google Scholar]
- Gruenberg JE, Griffith G, Howell KE. Characterization of the early endosome and putative endocytic carrier vesicles in vivo and with an assay of vesicle fusion in-vitro. J Cell Biol. 1989;108:1301–1316. doi: 10.1083/jcb.108.4.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenberg JE, Howell KE. An internalized transmembrane protein resides in a fusion-competent endosome for less than 5 min. Proc Natl Acad Sci USA. 1987;84:5758–5762. doi: 10.1073/pnas.84.16.5758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson PI, Roth R, Morisaki H, Jahn R, Heuser JE. Structure and conformational changes in NSF and its membrane receptor complexes visualized by quick-freeze/deep-etch electron microscopy. Cell. 1997;90:523–535. doi: 10.1016/s0092-8674(00)80512-7. [DOI] [PubMed] [Google Scholar]
- Heuser JE, Reese TS. Evidence for recycling of synaptic vesicle membrane during transmitter release at the frog neuromuscular junction. J Cell Biol. 1973;57:315–344. doi: 10.1083/jcb.57.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi H, et al. A novel rab5 GDP/GTP exchange factor complexed to rabaptin-5 links nucleotide exchange to effector recruitment and function. Cell. 1997;90:1149–1159. doi: 10.1016/s0092-8674(00)80380-3. [DOI] [PubMed] [Google Scholar]
- Jahn R, Hanson PI. Membrane fusion. SNAREs line up in new environment. Nature. 1998;393:14–15. doi: 10.1038/29871. [DOI] [PubMed] [Google Scholar]
- Jahn R, Schiebler W, Ouimet C, Greengard P. A 38,000-dalton membrane protein (p38) present in synaptic vesicles. Proc Natl Acad Sci USA. 1985;82:4137–4141. doi: 10.1073/pnas.82.12.4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo I, Harris HW, Amendt-Raduege AM, Majewski RR, Hammond TG. Rat kidney papilla contains abundant synaptobrevin protein that participates in the fusion of antidiuretic hormone-regulated water channel-containing endsosomes in-vitro. Proc Natl Acad Sci USA. 1995;92:1876–1880. doi: 10.1073/pnas.92.6.1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly RB. Storage and release of neurotransmitters. Cell. 1993;72(suppl):43–53. doi: 10.1016/s0092-8674(05)80027-3. [DOI] [PubMed] [Google Scholar]
- Koenig JH, Ikeda K. Disappearance and reformation of synaptic vesicle membrane upon transmitter release observed under reversible blockage of membrane retrieval. J Neurosci. 1989;9:3844–3860. doi: 10.1523/JNEUROSCI.09-11-03844.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lichtenstein Y, Desnos C, Faúndez V, Kelly RB, Clift-O’Grady L. Vesiculation and sorting from PC12-derived endosomes in-vitro. Proc Natl Acad Sci USA. 1998;95:11223–11228. doi: 10.1073/pnas.95.19.11223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link E, McMahon H, Fischer von Mollard G, Yamasaki S, Niemann H, Südhof TC, Jahn R. Cleavage of cellubrevin by tetanus toxin does not affect fusion of early endosomes. J Biol Chem. 1993;268:18423–18426. [PubMed] [Google Scholar]
- Linstedt AD, Kelly RB. Synaptophysin is sorted from endocytotic markers in neuroendocrine PC12 cells but not transfected fibroblasts. Neuron. 1991;7:309–317. doi: 10.1016/0896-6273(91)90269-6. [DOI] [PubMed] [Google Scholar]
- Mayorga LS, Beron W, Sarrouf MN, Colombo MI, Creutz C, Stahl PD. Calcium-dependent fusion among endosomes. J Biol Chem. 1994;269:30927–30934. [PubMed] [Google Scholar]
- Mayorga LS, Diaz R, Stahl PD. Plasma membrane-derived vesicles containing receptor-ligand complexes are fusogenic with early endosomes in a cell-free system. J Biol Chem. 1988;263:17213–17216. [PubMed] [Google Scholar]
- Montecucco C, Schiavo G. Structure and function of tetanus and botulinum neurotoxins. Q Rev Biophys. 1995;28:423–472. doi: 10.1017/s0033583500003292. [DOI] [PubMed] [Google Scholar]
- Murthy VN, Stevens CF. Synaptic vesicles retain their identity through the endocytic cycle. Nature. 1998;392:497–501. doi: 10.1038/33152. [DOI] [PubMed] [Google Scholar]
- Niemann H, Blasi J, Jahn R. Clostridial neurotoxins: new tools for dissecting exocytosis. Trends Cell Biol. 1994;4:179–185. doi: 10.1016/0962-8924(94)90203-8. [DOI] [PubMed] [Google Scholar]
- Otto H, Hanson PI, Jahn R. Assembly and disassembly of a ternary complex of synaptobrevin, syntaxin, and SNAP-25 in the membrane of synaptic vesicles. Proc Natl Acad Sci USA. 1997;94:6197–6201. doi: 10.1073/pnas.94.12.6197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters C, Mayer A. Ca2+/calmodulin signals the completion of docking and triggers a late step of vacuole fusion. Nature. 1998;396:575–580. doi: 10.1038/25133. [DOI] [PubMed] [Google Scholar]
- Pizzo P, Fasolato C, Pozzan F. Dynamic properties of an inositol 1,4,5-triphosphate- and thapsigargin-insensitive calcium pool in mammalian cell lines. J Cell Biol. 1997;136:355–366. doi: 10.1083/jcb.136.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prekeris R, Klumperman J, Chen YA, Scheller RH. Syntaxin 13 mediates cycling of plasma membrane proteins via tubulovesicular recycling endosomes. J Cell Biol. 1998;143:957–971. doi: 10.1083/jcb.143.4.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsh EF, Maniatis T. Molecular Cloning: a Laboratory Manual. 2nd ed. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Shi G, Faúndez V, Roos J, Dell’Angelica EC, Kelly RB. Neuroendocrine synaptic vesicles are formed in-vitro by both clathrin-dependent and clathrin-independent pathways. J Cell Biol. 1998;143:947–955. doi: 10.1083/jcb.143.4.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shupliakov O, Löw P, Grabs HG, Chen H, David C, Takei K, De Camilli P, Brodin L. Synaptic vesicle endocytosis impaired by disruption of dynamin-SH3 domain interactions. Science. 1997;276:259–263. doi: 10.1126/science.276.5310.259. [DOI] [PubMed] [Google Scholar]
- Simonsen A, Lippé R, Christoforidis S, Gaullier J-M, Brech A, Callaghan J, Toh B-H, Murphy C, Zerial M, Stenmark H. EEA1 links PI(3)K function to rab5 regulation of endosome fusion. Nature. 1998;394:494–498. doi: 10.1038/28879. [DOI] [PubMed] [Google Scholar]
- Stenmark H, Vitale G, Ullrich O, Zerial M. Rabaptin-5 is a direct effector of the small GTPase rab5 in endocytic membrane fusion. Cell. 1995;83:423–432. doi: 10.1016/0092-8674(95)90120-5. [DOI] [PubMed] [Google Scholar]
- Sullivan KMC, Busa WB, Wilson KL. Calcium mobilization is required for nuclear vesicle fusion in vitro: implications for membrane traffic and IP3 receptor function. Cell. 1993;73:1411–1422. doi: 10.1016/0092-8674(93)90366-x. [DOI] [PubMed] [Google Scholar]
- Tang BL, Tan AEH, Lim LK, Lee SS, Low DYH, Hong W. Syntaxin 12, a member of the syntaxin family localized to the endosome. J Biol Chem. 1998;273:6944–6950. doi: 10.1074/jbc.273.12.6944. [DOI] [PubMed] [Google Scholar]
- Tsien RW. New calcium indicators and buffers with high selectivity against magnesium and protons: design, synthesis, and properties of prototype structures. Biochemistry. 1980;19:2396–2404. doi: 10.1021/bi00552a018. [DOI] [PubMed] [Google Scholar]
- Ungermann C, Sato K, Wickner W. Defining the functions of trans-SNARE pairs. Nature. 1998;396:543–548. doi: 10.1038/25069. [DOI] [PubMed] [Google Scholar]
- Walch-Solimena C, Blasi J, Edelmann L, Chapman ER, Fischer von Mollard G, Jahn R. The t-SNAREs syntaxin 1 and SNAP-25 are present on organelles that participate in synaptic vesicle recycling. J Cell Biol. 1995;128:637–645. doi: 10.1083/jcb.128.4.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldron RT, Short AD, Gill DL. Thapsigargin-resistent intracellular calcium pumps. J Biol Chem. 1995;270:11955–11951. doi: 10.1074/jbc.270.20.11955. [DOI] [PubMed] [Google Scholar]
- Wong SH, Xu Y, Zhang T, Hong W. Syntaxin 7, a novel syntaxin member associated with the early endosomal compartment. J Biol Chem. 1998a;273:375–380. doi: 10.1074/jbc.273.1.375. [DOI] [PubMed] [Google Scholar]
- Wong SH, Zhang T, Xu Y, Subramaniam VN, Griffiths G, Hong W. Endobrevin, a novel synaptobrevin/VAMP-like protein preferentially associated with the early endosome. Mol Biol Cell. 1998b;9:1549–1563. doi: 10.1091/mbc.9.6.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodman PG, Warren G. Isolation of functional, coated, endocytic vesicles. J Cell Biol. 1991;112:1133–1141. doi: 10.1083/jcb.112.6.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Gonzalez, Prekeris R, Steegmaier M, Advani RJ, Scheller R. SNARE interactions are not selective. J Biol Chem. 1999;274:5649–5653. doi: 10.1074/jbc.274.9.5649. [DOI] [PubMed] [Google Scholar]