Abstract
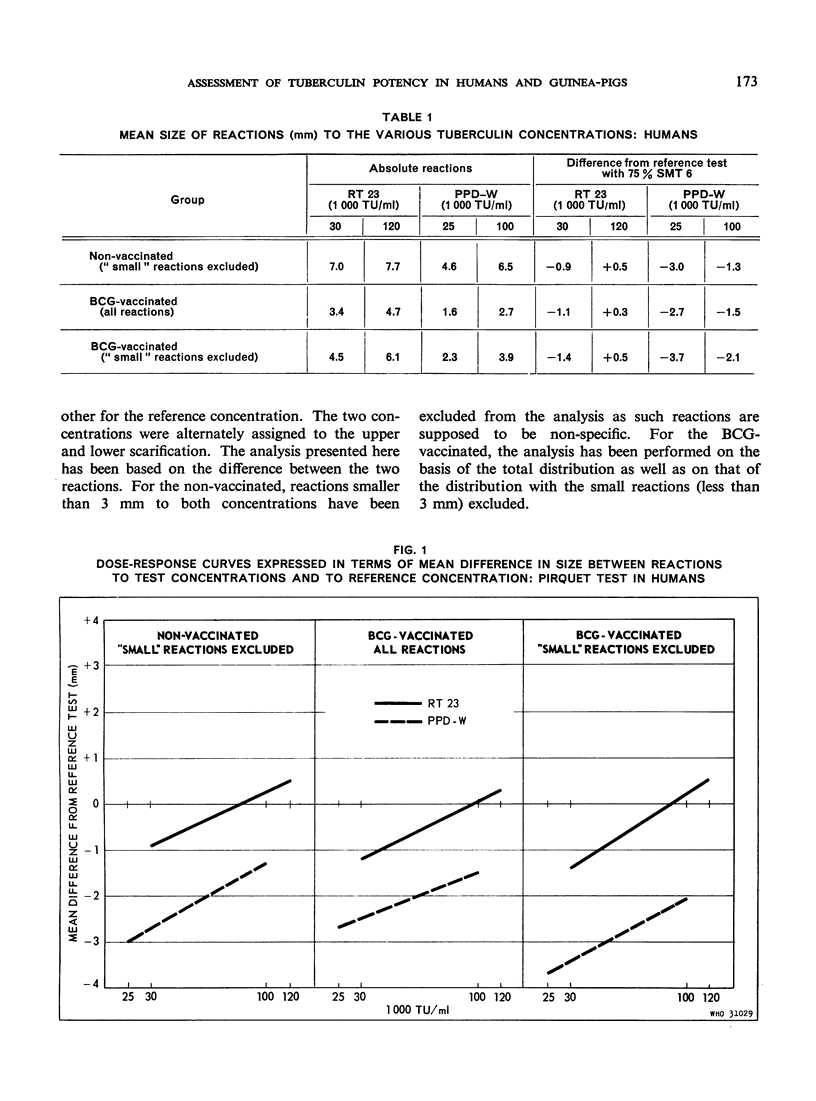
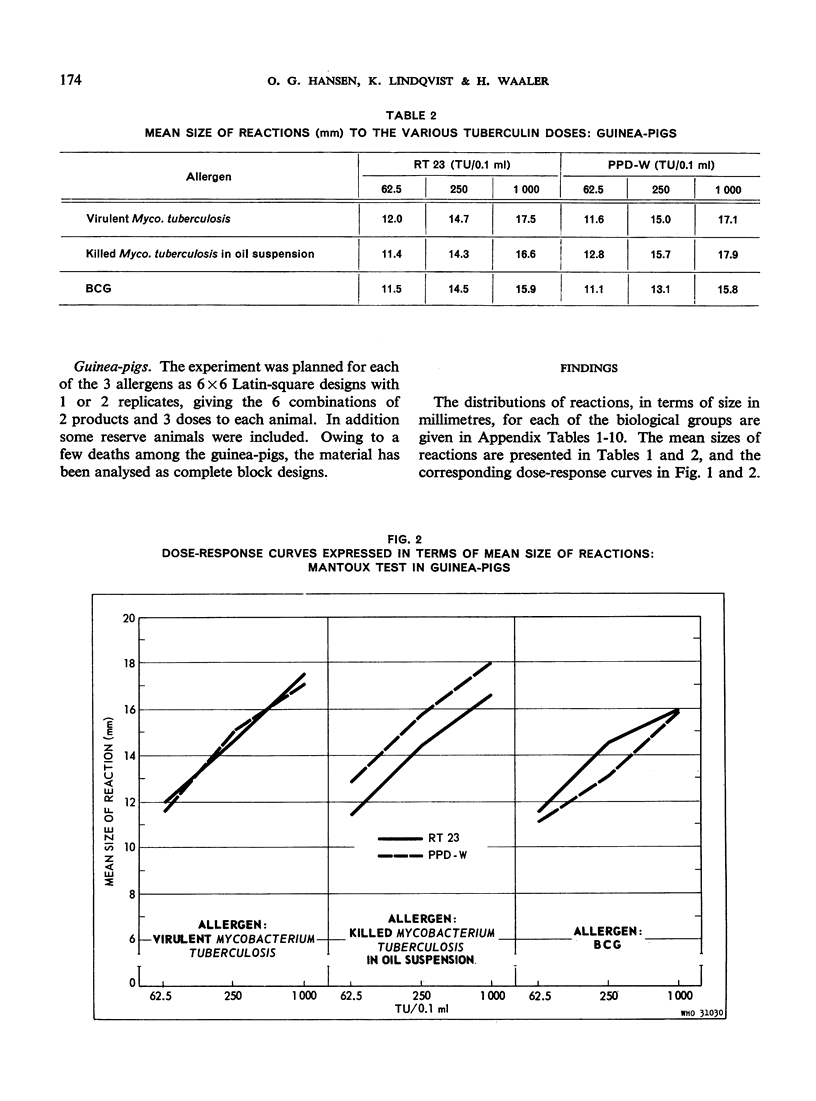
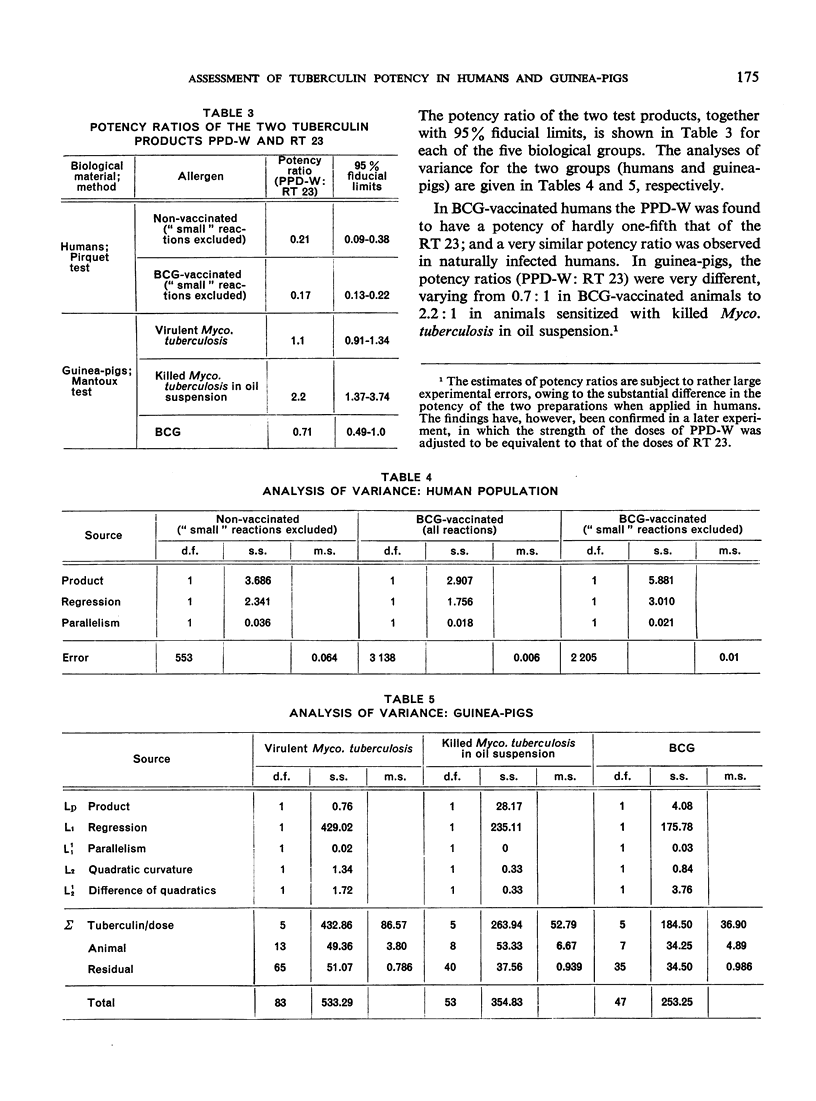
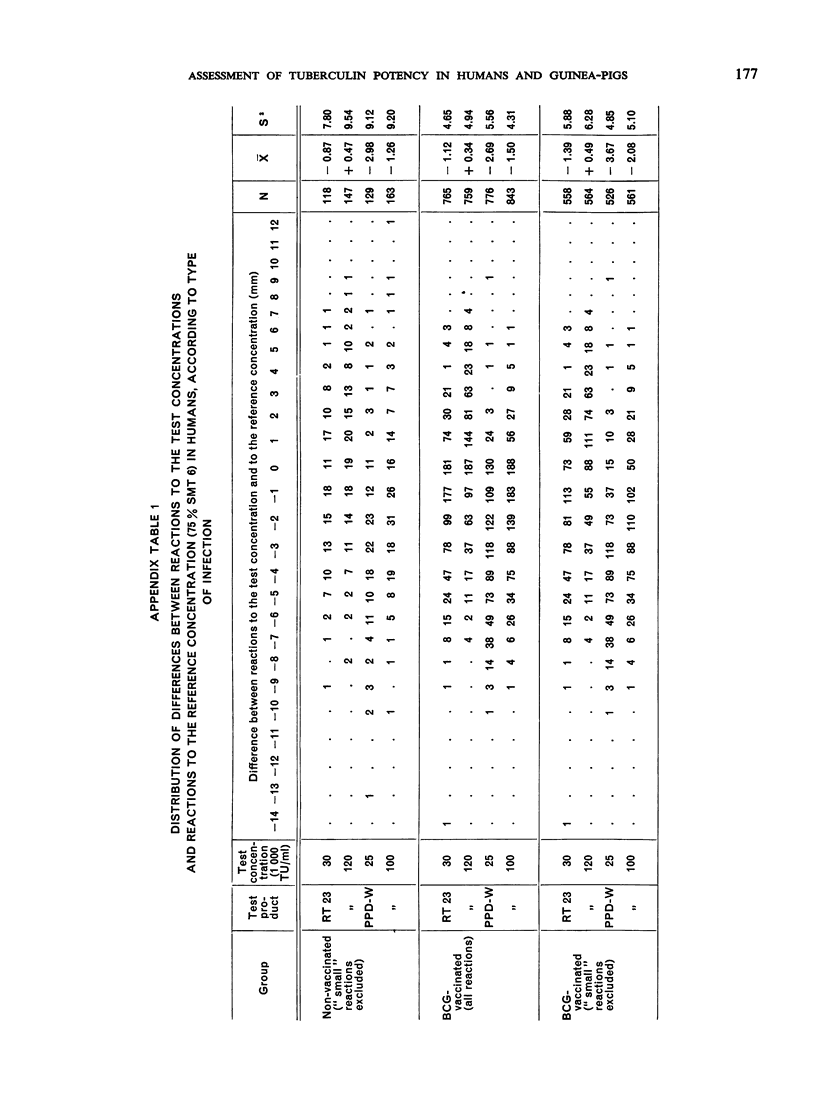
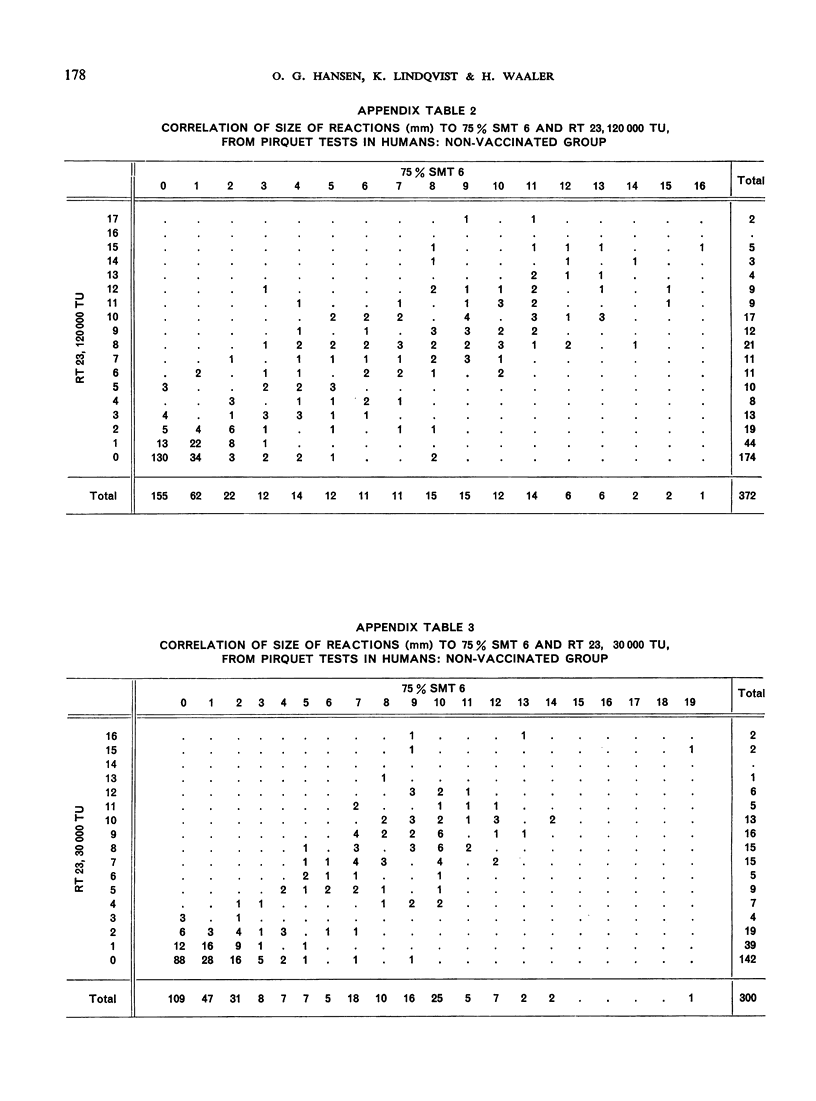
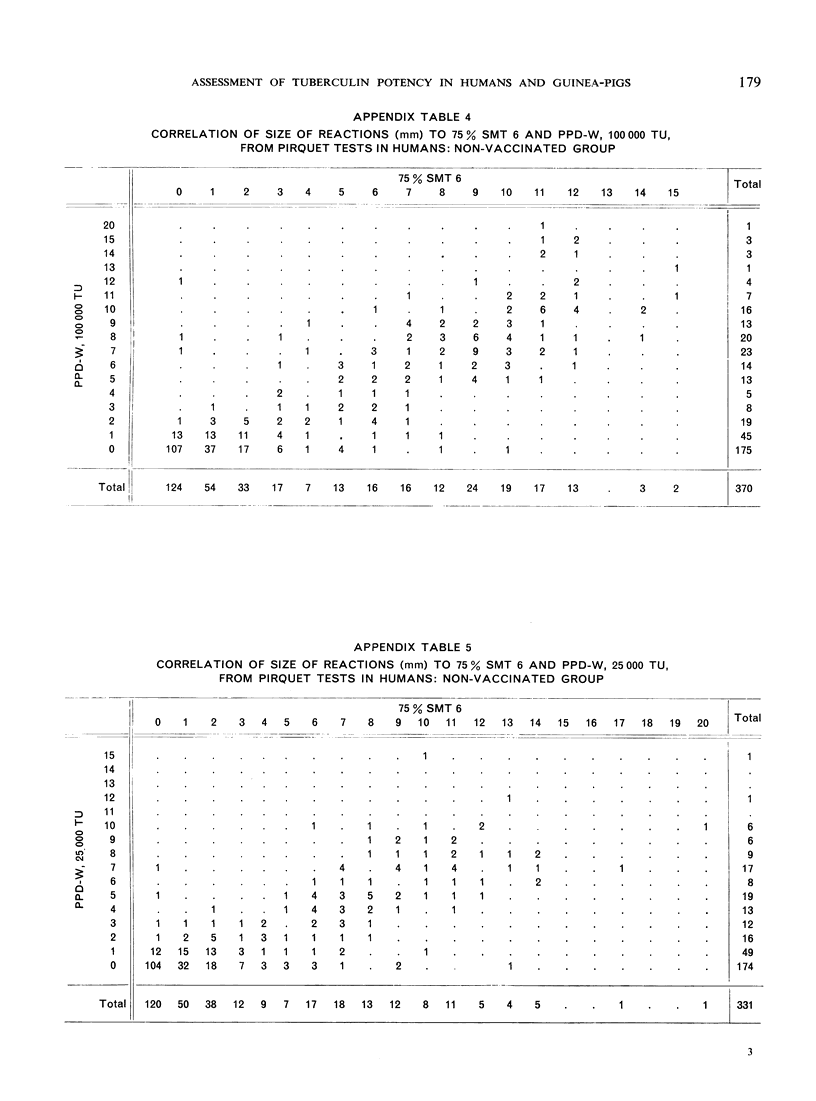
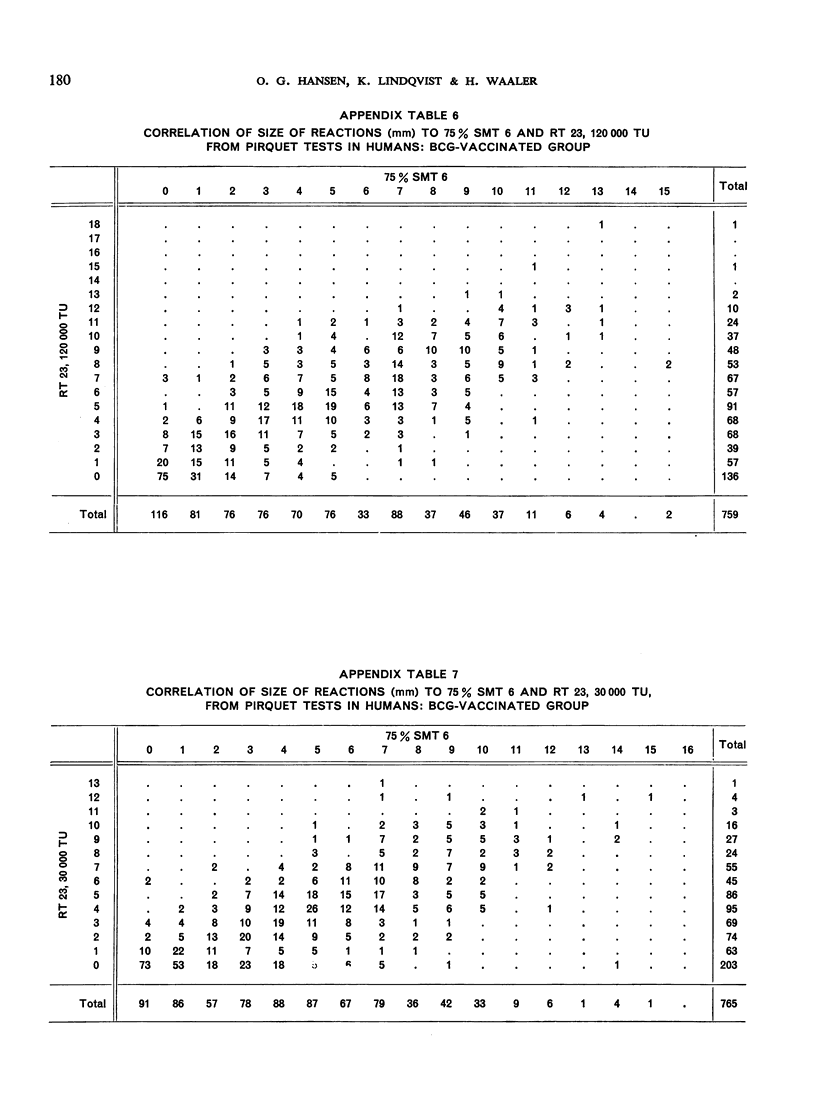
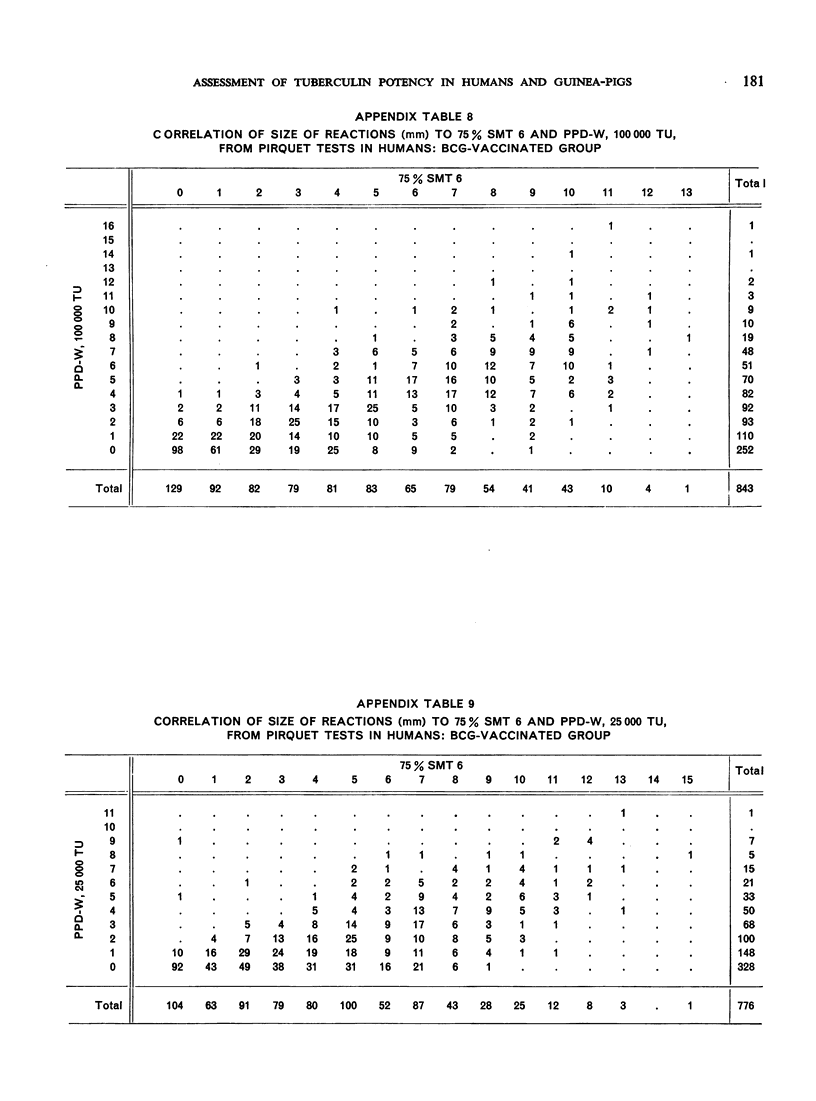
The biological effects of tuberculin in an organism infected with tubercle bacilli are due to an unknown number of different substances, which are present in varying concentrations in different tuberculin preparations. Consequently, the response to tuberculin tests with various products is likely to vary with the biological material, the test technique, the tuberculin dose, etc., and potency estimates of tuberculin products will depend to a considerable extent on how the assessment is made. The present paper describes an experiment in which this dependence was demonstrated in the case of two purified protein derivative tuberculin preparations (RT 23 and PPD-Weybridge). The potency ratio of these products was found to vary widely according to whether it was determined in humans by the Pirquet test or in guinea-pigs by the Mantoux test, and the authors therefore conclude that tuberculin products must be assessed under the conditions in which they will subsequently be used.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- GULD J., BENTZON M. W., BLEIKER M. A., GRIEP W. A., MAGNUSSON M., WAALER H. Standardization of a new batch of purified tuberculin (PPD) intended for international use. Bull World Health Organ. 1958;19(5):845–951. [PMC free article] [PubMed] [Google Scholar]
- LONG D. A., MILES A. A., PERRY W. L. The assay of tuberculin. Bull World Health Organ. 1954;10(6):989–1002. [PMC free article] [PubMed] [Google Scholar]


