Abstract
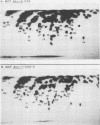
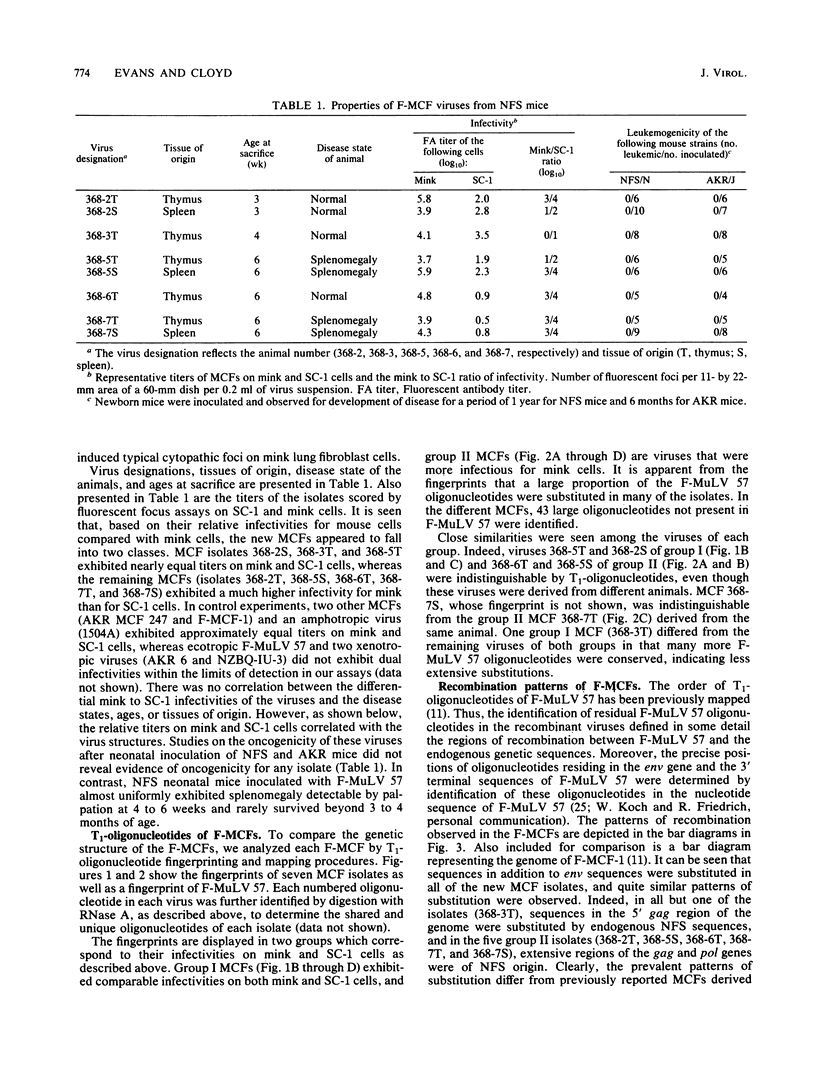
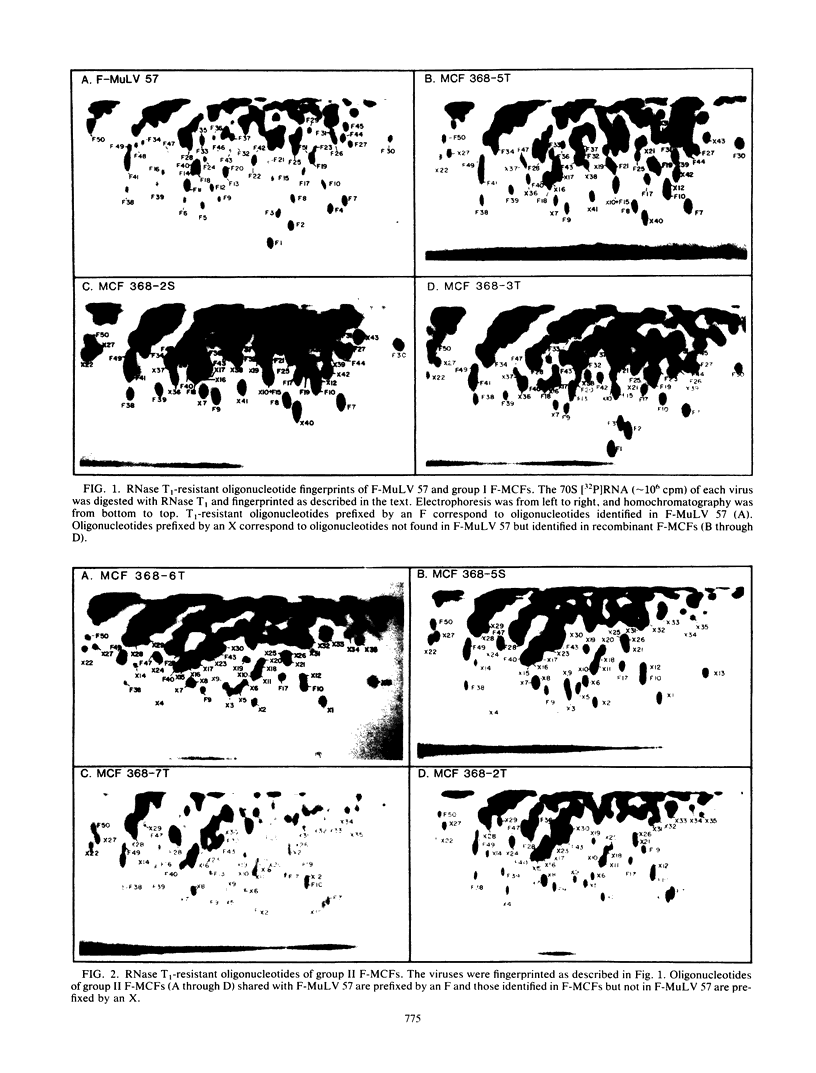
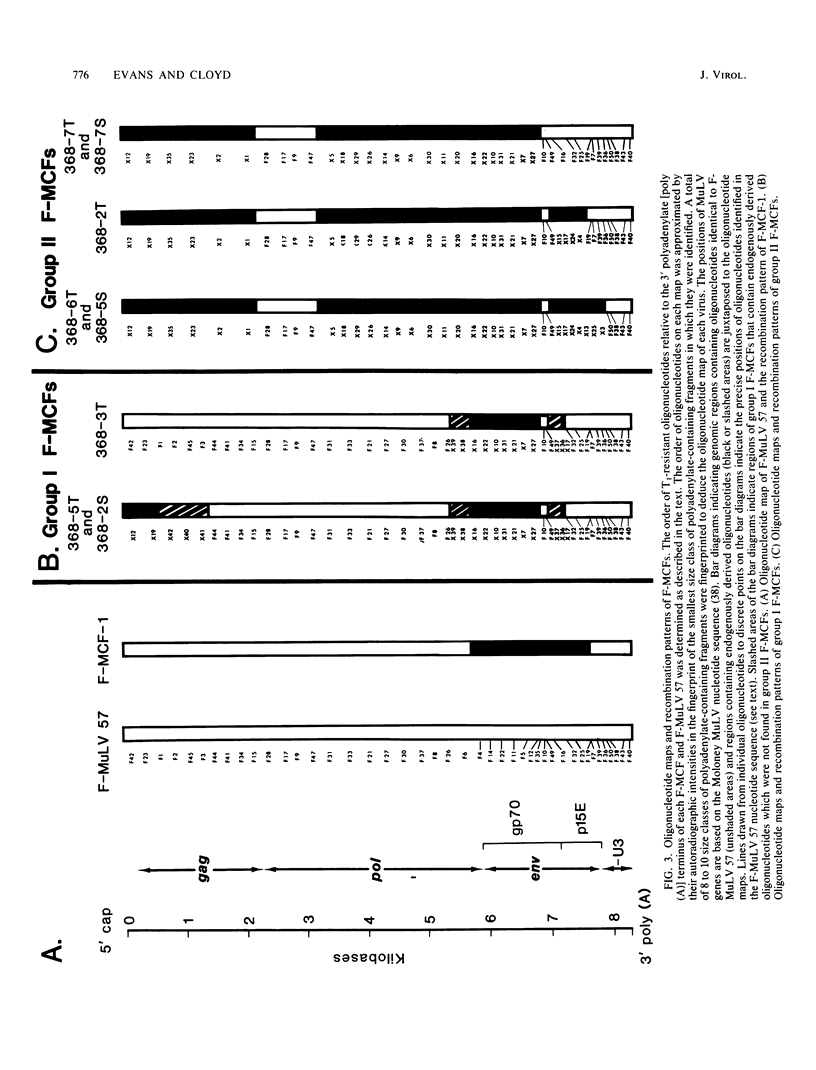
A family of recombinant mink cell focus-forming viruses (MCF) was derived by inoculation of NFS mice with a Friend murine leukemia virus, and their genomes were analyzed by RNase T1-resistant oligonucleotide fingerprinting. The viruses were obtained from the thymuses and spleens of preleukemic and leukemic animals and were evaluated for dualtropism and oncogenicity. All these isolates induced cytopathic foci on mink cells but could be classified into two groups based on their relative infectivities for SC-1 (mouse) or mink (ATCC CCL64) cells. One group of Friend MCFs (F-MCFs) (group I) exhibited approximately equal infectivities for SC-1 and mink cells, whereas a second group (group II) infected mink cells 1,000- to 10,000-fold more efficiently than SC-1 cells. Structural analyses of the F-MCFs revealed that group I and group II viruses correlated with recombination of Friend murine leukemia virus with two distinct, but closely related, endogenous NFS proviral sequences. No correlation was found between the type of F-MCF and the tissue of origin or the disease state of the animal. Furthermore, none of the F-MCF isolates were found to be oncogenic in NFS/N or AKR/J mice. F-MCFs of both groups underwent extensive substitution of ecotropic sequences, involving much of the gag and env genes of group I F-MCFs and most of the gag, pol, and env genes of group II F-MCFs. All F-MCF isolates retained the 3' terminal U3 region of Friend murine leukemia virus. Comparison of the RNAs of the F-MCFs with RNAs of MCFs derived from NFS.Akv-1 or NFS.Akv-2 mice indicated that the F-MCFs were derived from NFS proviral sequences which are distinct from the sequences contained in NFS.Akv MCF isolates. This result suggested that recombination with particular endogenous proviral sequences to generate MCFs may be highly specific for a given murine leukemia virus.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi A., Sakai K., Ishimoto A. Isolation and characterization of mink cell focus-inducing murine leukemia viruses with xenotropic host range from mouse strain SL. J Virol. 1983 Jan;45(1):447–451. doi: 10.1128/jvi.45.1.447-451.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosselman R. A., Van Griensven L. J., Vogt M., Verma I. M. Genome organization of retroviruses. VI. Heteroduplex analysis of ecotropic and xenotropic sequences of moloney mink cell focus-inducing viral RNA obtained from either a cloned isolate or a thymoma cell line. J Virol. 1979 Dec;32(3):968–978. doi: 10.1128/jvi.32.3.968-978.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosselman R. A., van Straaten F., Van Beveren C., Verma I. M., Vogt M. Analysis of the env gene of a molecularly cloned and biologically active Moloney mink cell focus-forming proviral DNA. J Virol. 1982 Oct;44(1):19–31. doi: 10.1128/jvi.44.1.19-31.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Cloyd M. W., Linemeyer D. L., Lander M. R., Rands E., Lowy D. R. Cellular origin and role of mink cell focus-forming viruses in murine thymic lymphomas. Nature. 1982 Jan 7;295(5844):25–31. doi: 10.1038/295025a0. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Lander M. R., Gupta S., Rands E., Lowy D. R. Origin of mink cytopathic focus-forming (MCF) viruses:comparison with ecotropic and xenotropic murine leukemia virus genomes. Virology. 1981 Sep;113(2):465–483. doi: 10.1016/0042-6822(81)90175-6. [DOI] [PubMed] [Google Scholar]
- Chien Y. H., Verma I. M., Shih T. Y., Scolnick E. M., Davidson N. Heteroduplex analysis of the sequence relations between the RNAs of mink cell focus-inducing and murine leukemia viruses. J Virol. 1978 Oct;28(1):352–360. doi: 10.1128/jvi.28.1.352-360.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloyd M. W., Hartley J. W., Rowe W. P. Lymphomagenicity of recombinant mink cell focus-inducing murine leukemia viruses. J Exp Med. 1980 Mar 1;151(3):542–552. doi: 10.1084/jem.151.3.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue D. J., Rothenberg E., Hopkins N., Baltimore D., Sharp P. A. Heteroduplex analysis of the nonhomology region between Moloney MuLV and the dual host range derivative HIX virus. Cell. 1978 Aug;14(4):959–970. doi: 10.1016/0092-8674(78)90350-1. [DOI] [PubMed] [Google Scholar]
- Elder J. H., Gautsch J. W., Jensen F. C., Lerner R. A., Hartley J. W., Rowe W. P. Biochemical evidence that MCF murine leukemia viruses are envelope (env) gene recombinants. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4676–4680. doi: 10.1073/pnas.74.10.4676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans L. H., Duesberg P. H., Troxler D. H., Scolnick E. M. Spleen focus-forming Friend virus: identification of genomic RNA and its relationship to helper virus RNA. J Virol. 1979 Jul;31(1):133–146. doi: 10.1128/jvi.31.1.133-146.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans L., Nunn M., Duesberg P. H., Troxler D., Scolnick E. RNAs of defective and nondefective components of Friend anemia and polycythemia virus strains identified and compared. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):823–835. doi: 10.1101/sqb.1980.044.01.087. [DOI] [PubMed] [Google Scholar]
- Faller D. V., Hopkins N. T1 oligonucleotide maps of Moloney and HIX murine leukemia viruses. Virology. 1978 Oct 15;90(2):265–273. doi: 10.1016/0042-6822(78)90310-0. [DOI] [PubMed] [Google Scholar]
- Fischinger P. J., Ihle J. N., de Noronha F., Bolognesi D. P. Oncogenic and immunogenic potential of cloned HIX virus in mice and cats. Med Microbiol Immunol. 1977;164(1-3):119–129. doi: 10.1007/BF02121308. [DOI] [PubMed] [Google Scholar]
- Fischinger P. J., Nomura S., Bolognesi D. P. A novel murine oncornavirus with dual eco- and xenotropic properties. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5150–5155. doi: 10.1073/pnas.72.12.5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green N., Hiai H., Elder J. H., Schwartz R. S., Khiroya R. H., Thomas C. Y., Tsichlis P. N., Coffin J. M. Expression of leukemogenic recombinant viruses associated with a recessive gene in HRS/J mice. J Exp Med. 1980 Aug 1;152(2):249–264. doi: 10.1084/jem.152.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas M. Leukemogenic activity of thymotropic, ecotropic, and xenotropic radiation leukemia virus isolates. J Virol. 1978 Mar;25(3):705–709. doi: 10.1128/jvi.25.3.705-709.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P. Clonal cells lines from a feral mouse embryo which lack host-range restrictions for murine leukemia viruses. Virology. 1975 May;65(1):128–134. doi: 10.1016/0042-6822(75)90013-6. [DOI] [PubMed] [Google Scholar]
- Hartley J. W., Wolford N. K., Old L. J., Rowe W. P. A new class of murine leukemia virus associated with development of spontaneous lymphomas. Proc Natl Acad Sci U S A. 1977 Feb;74(2):789–792. doi: 10.1073/pnas.74.2.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson I. C., Lieber M. M., Todaro G. J. Mink cell line Mv 1 Lu (CCL 64). Focus formation and the generation of "nonproducer" transformed cell lines with murine and feline sarcoma viruses. Virology. 1974 Jul;60(1):282–287. doi: 10.1016/0042-6822(74)90386-9. [DOI] [PubMed] [Google Scholar]
- Hiai H., Morrissey P., Khiroya R., Schwartz R. S. Selective expression of xenotropic virus in congenic HRS/J (hairless) mice. Nature. 1977 Nov 17;270(5634):247–249. doi: 10.1038/270247a0. [DOI] [PubMed] [Google Scholar]
- Hoggan M. D., Buckler C. E., Sears J. F., Chan H. W., Rowe W. P., Martin M. A. Internal organization of endogenous proviral DNAs of xenotropic murine leukemia viruses. J Virol. 1982 Jul;43(1):8–17. doi: 10.1128/jvi.43.1.8-17.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoggan M. D., Buckler C. E., Sears J. F., Rowe W. P., Martin M. A. Organization and stability of endogenous xenotropic murine leukemia virus proviral DNA in mouse genomes. J Virol. 1983 Jan;45(1):473–477. doi: 10.1128/jvi.45.1.473-477.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimoto A., Adachi A., Sakai K., Yorifuji T., Tsuruta S. Rapid emergence of mink cell focus-forming (MCF) virus in various mice infected with NB-tropic friend virus. Virology. 1981 Sep;113(2):644–655. doi: 10.1016/0042-6822(81)90193-8. [DOI] [PubMed] [Google Scholar]
- Khan A. S., Rowe W. P., Martin M. A. Cloning of endogenous murine leukemia virus-related sequences from chromosomal DNA of BALB/c and AKR/J mice: identification of an env progenitor of AKR-247 mink cell focus-forming proviral DNA. J Virol. 1982 Nov;44(2):625–636. doi: 10.1128/jvi.44.2.625-636.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch W., Hunsmann G., Friedrich R. Nucleotide sequence of the envelope gene of Friend murine leukemia virus. J Virol. 1983 Jan;45(1):1–9. doi: 10.1128/jvi.45.1.1-9.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lung M. L., Hartley J. W., Rowe W. P., Hopkins N. H. Large RNase T1-resistant oligonucleotides encoding p15E and the U3 region of the long terminal repeat distinguish two biological classes of mink cell focus-forming type C viruses of inbred mice. J Virol. 1983 Jan;45(1):275–290. doi: 10.1128/jvi.45.1.275-290.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOLONEY J. B. Properties of a leukemia virus. Natl Cancer Inst Monogr. 1960 Sep;4:7–37. [PubMed] [Google Scholar]
- MacDonald M. E., Mak T. W., Bernstein A. Erythroleukemia induction by replication-competent type C viruses cloned from the anemia- and polycythemia-inducing isolates of Friend leukemia virus. J Exp Med. 1980 Jun 1;151(6):1493–1503. doi: 10.1084/jem.151.6.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowinski R. C., Hays E. F. Oncogenicity of AKR endogenous leukemia viruses. J Virol. 1978 Jul;27(1):13–18. doi: 10.1128/jvi.27.1.13-18.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliff A. I., Hager G. L., Chang E. H., Scolnick E. M., Chan H. W., Lowy D. R. Transfection of molecularly cloned Friend murine leukemia virus DNA yields a highly leukemogenic helper-independent type C virus. J Virol. 1980 Jan;33(1):475–486. doi: 10.1128/jvi.33.1.475-486.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommelaere J., Faller D. V., Hopkins N. Characterization and mapping of RNase T1-resistant oligonucleotides derived from the genomes of Akv and MCF murine leukemia viruses. Proc Natl Acad Sci U S A. 1978 Jan;75(1):495–499. doi: 10.1073/pnas.75.1.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe W. P., Cloyd M. W., Hartley J. W. Status of the association of mink cell focus-forming viruses with leukemogenesis. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):1265–1268. doi: 10.1101/sqb.1980.044.01.137. [DOI] [PubMed] [Google Scholar]
- Rowe W. P., Kozak C. A. Germ-line reinsertions of AKR murine leukemia virus genomes in Akv-1 congenic mice. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4871–4874. doi: 10.1073/pnas.77.8.4871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe W. P. Leukemia virus genomes in the chromosomal DNA of the mouse. Harvey Lect. 1978;71:173–192. [PubMed] [Google Scholar]
- Rowe W. P., Pugh W. E., Hartley J. W. Plaque assay techniques for murine leukemia viruses. Virology. 1970 Dec;42(4):1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- Ruscetti S., Davis L., Feild J., Oliff A. Friend murine leukemia virus-induced leukemia is associated with the formation of mink cell focus-inducing viruses and is blocked in mice expressing endogenous mink cell focus-inducing xenotropic viral envelope genes. J Exp Med. 1981 Sep 1;154(3):907–920. doi: 10.1084/jem.154.3.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih T. Y., Weeks M. O., Troxler D. H., Coffin J. M., Scolnick E. M. Mapping host range-specific oligonucleotides within genomes of the ecotropic and mink cell focus-inducing strains of Moloney murine leukemia virus. J Virol. 1978 Apr;26(1):71–83. doi: 10.1128/jvi.26.1.71-83.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinnick T. M., Lerner R. A., Sutcliffe J. G. Nucleotide sequence of Moloney murine leukaemia virus. Nature. 1981 Oct 15;293(5833):543–548. doi: 10.1038/293543a0. [DOI] [PubMed] [Google Scholar]
- Steffen D. L., Mural R., Cowing D., Mielcarz J., Young J., Roblin R. Most of the murine leukemia virus sequences in the DNA of NIH/swiss mice consist of two closely related proviruses, each repeated several times. J Virol. 1982 Jul;43(1):127–135. doi: 10.1128/jvi.43.1.127-135.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C. Y., Coffin J. M. Genetic alterations of RNA leukemia viruses associated with the development of spontaneous thymic leukemia in AKR/J mice. J Virol. 1982 Aug;43(2):416–426. doi: 10.1128/jvi.43.2.416-426.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troxler D. H., Scolnick E. M. Rapid leukemia induced by cloned friend strain of replicating murine type-C virus. Association with induction of xenotropic-related RNA sequences contained in spleen focus-forming virus. Virology. 1978 Mar;85(1):17–27. doi: 10.1016/0042-6822(78)90408-7. [DOI] [PubMed] [Google Scholar]
- Vogt M. Properties of "mink cell focus-inducing" (MCF) virus isolated from spontaneous lymphoma lines of BALB/c mice carrying Moloney leukemia virus as an endogenous virus. Virology. 1979 Feb;93(1):226–236. doi: 10.1016/0042-6822(79)90290-3. [DOI] [PubMed] [Google Scholar]
- van Griensven L. J., Vogt M. Rauscher "mink cell focus-inducing" (MCF) virus causes erythroleukemia in mice: its isolation and properties. Virology. 1980 Mar;101(2):376–388. doi: 10.1016/0042-6822(80)90451-1. [DOI] [PubMed] [Google Scholar]
- van der Putten H., Quint W., van Raaij J., Maandag E. R., Verma I. M., Berns A. M-MuLV-induced leukemogenesis: integration and structure of recombinant proviruses in tumors. Cell. 1981 Jun;24(3):729–739. doi: 10.1016/0092-8674(81)90099-4. [DOI] [PubMed] [Google Scholar]