Abstract
At the request of the Ministry of Health of the USSR, a controlled field trial of a polyvaccine containing typhoid, paratyphoid and dysentery antigens and a purified tetanus toxoid was undertaken in 1958. The main object of this trial, which was carried out over a 10-month period simultaneously in four localities, was to determine the efficacy of the typhoid component of the polyvaccine.
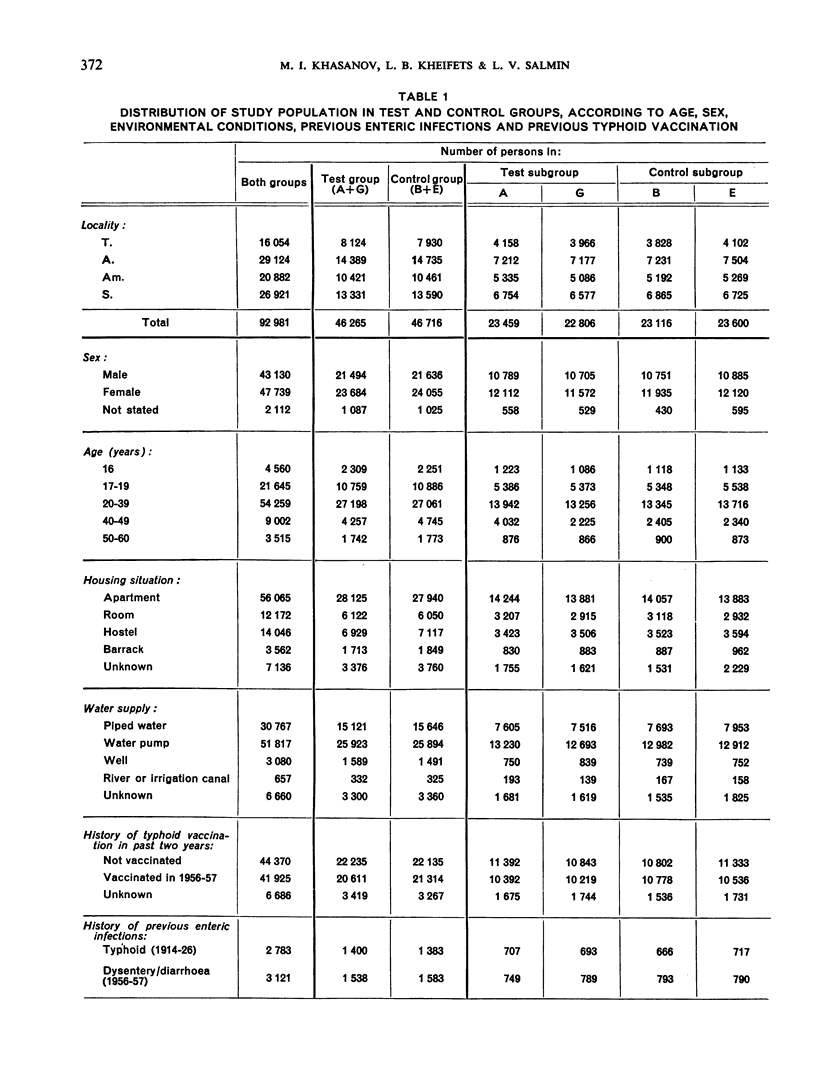
The study population comprised over 90 000 individuals of 16-60 years of age. These were divided into two approximately equal groups, one of which received an injection of the polyvaccine under test and the other an injection of a preparation containing only the purified tetanus toxoid.
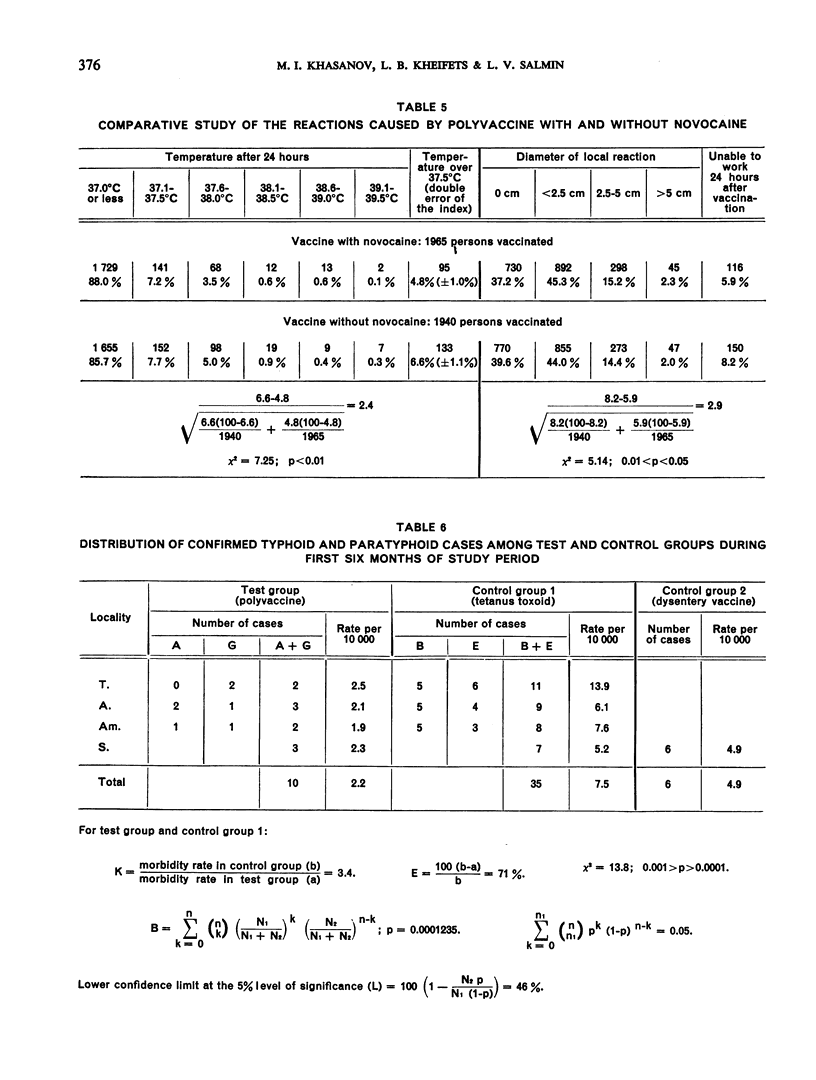
The difference between the incidence of typhoid fever in the test group and that in the control group during the study period was statistically significant, and it was therefore concluded that a single injection of the polyvaccine afforded some protection against typhoid.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COCKBURN W. C. Large-scale field trials of active immunizing agents; with special reference to vaccination against pertussis. Bull World Health Organ. 1955;13(3):395–407. [PMC free article] [PubMed] [Google Scholar]
- HILL A. B. The clinical trial. Br Med Bull. 1951;7(4):278–282. doi: 10.1093/oxfordjournals.bmb.a073919. [DOI] [PubMed] [Google Scholar]


