Abstract
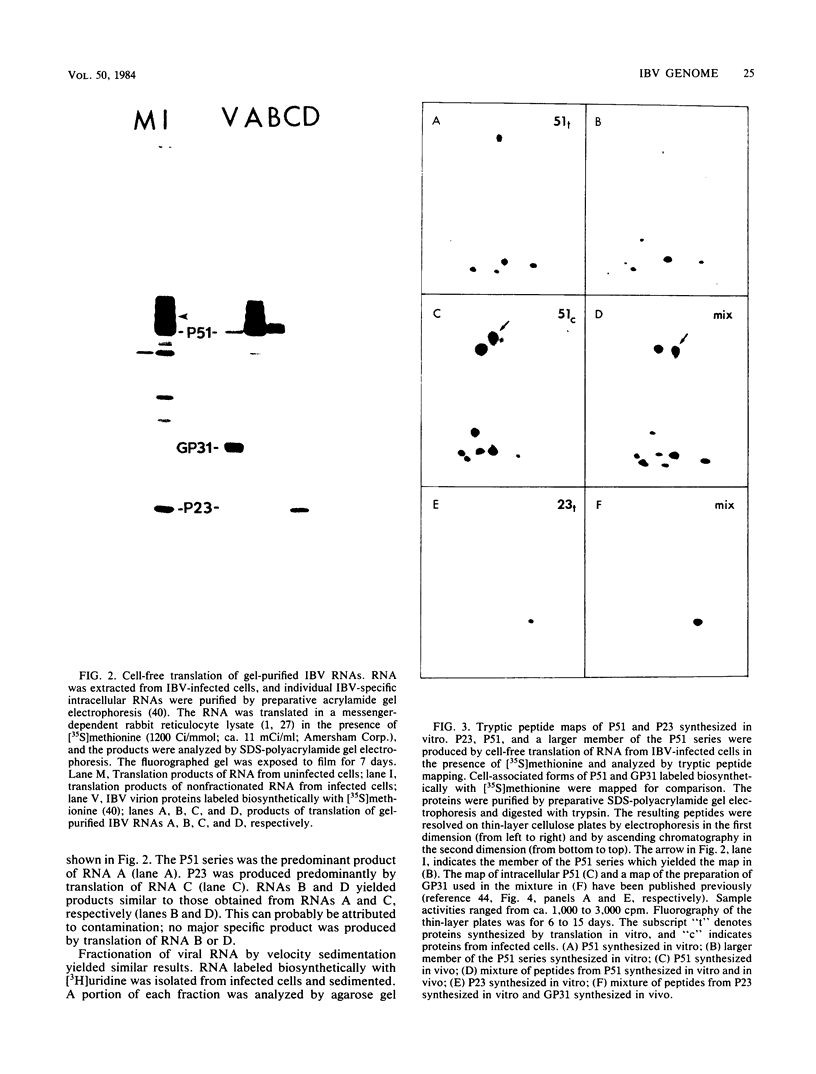
Six overlapping viral RNAs are synthesized in cells infected with the avian coronavirus infectious bronchitis virus (IBV). These RNAs contain a 3'-coterminal nested sequence set and were assumed to be viral mRNAs. The seven major IBV virion proteins are all produced by processing of three polypeptides of ca. 23, 51, and 115 kilodaltons. These are the core polypeptides of the small membrane proteins, the nucleocapsid protein, and the 155-kilodalton precursor to the large membrane proteins GP90 and GP84, respectively. To determine which mRNAs specify these polypeptides, we isolated RNA from infected cells and translated it in a messenger-dependent rabbit reticulocyte lysate. Proteins of 23, 51, and 110 kilodaltons were produced. Two-dimensional tryptic peptide mapping demonstrated that these proteins were closely related to the major virion proteins. Fractionation of the RNA before cell-free translation permitted the correlation of messenger activities for synthesis of the proteins with the presence of specific mRNAs. We found that the smallest RNA, RNA A, directs the synthesis of P51, the nucleocapsid protein. RNA C, which contains the sequences of RNA A, directs the synthesis of the small membrane protein P23. RNA E directs the synthesis of the large virion glycoproteins. These results supported a model in which only the unique 5'-terminal domain of each IBV mRNA is active in translation and enabled us to localize genes for virion proteins on the IBV genome.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beemon K., Hunter T. Characterization of Rous sarcoma virus src gene products synthesized in vitro. J Virol. 1978 Nov;28(2):551–566. doi: 10.1128/jvi.28.2.551-566.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonatti S., Sonenberg N., Shatkin A. J., Cancedda R. Restricted initiation of protein synthesis on the potentially polycistronic Sindbis virus 42 S RNA. J Biol Chem. 1980 Dec 10;255(23):11473–11477. [PubMed] [Google Scholar]
- Bonner W. M., Stedman J. D. Efficient fluorography of 3H and 14C on thin layers. Anal Biochem. 1978 Aug 15;89(1):247–256. doi: 10.1016/0003-2697(78)90747-9. [DOI] [PubMed] [Google Scholar]
- Cavanagh D. Structural polypeptides of coronavirus IBV. J Gen Virol. 1981 Mar;53(Pt 1):93–103. doi: 10.1099/0022-1317-53-1-93. [DOI] [PubMed] [Google Scholar]
- Cheley S., Anderson R. Cellular synthesis and modification of murine hepatitis virus polypeptides. J Gen Virol. 1981 Jun;54(Pt 2):301–311. doi: 10.1099/0022-1317-54-2-301. [DOI] [PubMed] [Google Scholar]
- Cheley S., Anderson R., Cupples M. J., Chan E. C., Morris V. L. Intracellular murine hepatitis virus-specific RNAs contain common sequences. Virology. 1981 Jul 30;112(2):596–604. doi: 10.1016/0042-6822(81)90305-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies H. A., Dourmashkin R. R., Macnaughton M. R. Ribonucleoprotein of avian infectious bronchitis virus. J Gen Virol. 1981 Mar;53(Pt 1):67–74. doi: 10.1099/0022-1317-53-1-67. [DOI] [PubMed] [Google Scholar]
- Goelet P., Karn J. Tobacco mosaic virus induces the synthesis of a family of 3' coterminal messenger RNAs and their complements. J Mol Biol. 1982 Jan 25;154(3):541–550. doi: 10.1016/s0022-2836(82)80013-2. [DOI] [PubMed] [Google Scholar]
- Holmes K. V., Doller E. W., Behnke J. N. Analysis of the functions of coronavirus glycoproteins by differential inhibition of synthesis with tunicamycin. Adv Exp Med Biol. 1981;142:133–142. doi: 10.1007/978-1-4757-0456-3_11. [DOI] [PubMed] [Google Scholar]
- Holmes K. V., Doller E. W., Sturman L. S. Tunicamycin resistant glycosylation of coronavirus glycoprotein: demonstration of a novel type of viral glycoprotein. Virology. 1981 Dec;115(2):334–344. doi: 10.1016/0042-6822(81)90115-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Jackson R., Zimmern D. Multiple proteins and subgenomic mRNAs may be derived from a single open reading frame on tobacco mosaic virus RNA. Nucleic Acids Res. 1983 Feb 11;11(3):801–821. doi: 10.1093/nar/11.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy S. I. Sequence relationships between the genome and the intracellular RNA species of standard and defective-interfering Semliki Forest virus. J Mol Biol. 1976 Dec;108(2):491–511. doi: 10.1016/s0022-2836(76)80132-5. [DOI] [PubMed] [Google Scholar]
- Kozak M. Mechanism of mRNA recognition by eukaryotic ribosomes during initiation of protein synthesis. Curr Top Microbiol Immunol. 1981;93:81–123. doi: 10.1007/978-3-642-68123-3_5. [DOI] [PubMed] [Google Scholar]
- Lai M. M., Brayton P. R., Armen R. C., Patton C. D., Pugh C., Stohlman S. A. Mouse hepatitis virus A59: mRNA structure and genetic localization of the sequence divergence from hepatotropic strain MHV-3. J Virol. 1981 Sep;39(3):823–834. doi: 10.1128/jvi.39.3.823-834.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanser J. A., Howard C. R. The polypeptides of infectious bronchitis virus (IBV-41 strain). J Gen Virol. 1980 Feb;46(2):349–361. doi: 10.1099/0022-1317-46-2-349. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Enhanced autoradiographic detection of 32P and 125I using intensifying screens and hypersensitized film. FEBS Lett. 1977 Oct 15;82(2):314–316. doi: 10.1016/0014-5793(77)80609-1. [DOI] [PubMed] [Google Scholar]
- Leibowitz J. L., DeVries J. R., Haspel M. V. Genetic analysis of murine hepatitis virus strain JHM. J Virol. 1982 Jun;42(3):1080–1087. doi: 10.1128/jvi.42.3.1080-1087.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibowitz J. L., Weiss S. R., Paavola E., Bond C. W. Cell-free translation of murine coronavirus RNA. J Virol. 1982 Sep;43(3):905–913. doi: 10.1128/jvi.43.3.905-913.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibowitz J. L., Wilhelmsen K. C., Bond C. W. The virus-specific intracellular RNA species of two murine coronaviruses: MHV-a59 and MHV-JHM. Virology. 1981 Oct 15;114(1):39–51. doi: 10.1016/0042-6822(81)90250-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomniczi B., Kennedy I. Genome of infectious bronchitis virus. J Virol. 1977 Oct;24(1):99–107. doi: 10.1128/jvi.24.1.99-107.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomniczi B., Morser J. Polypeptides of infectious bronchitis virus. I. Polypeptides of the virion. J Gen Virol. 1981 Jul;55(Pt 1):155–164. doi: 10.1099/0022-1317-55-1-155. [DOI] [PubMed] [Google Scholar]
- Macnaughton M. R., Madge M. H., Davies H. A., Dourmashkin R. R. Polypeptides of the surface projections and the ribonucleoprotein of avian infectious bronchitis virus. J Virol. 1977 Dec;24(3):821–825. doi: 10.1128/jvi.24.3.821-825.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macnaughton M. R., Madge M. H. The characterisation of the virion RNA of avian infectious bronchitis virus. FEBS Lett. 1977 May 15;77(2):311–313. doi: 10.1016/0014-5793(77)80258-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinkoth J., Kennedy S. I. Semliki forest virus persistence in mouse L929 cells. Virology. 1980 Jan 15;100(1):141–155. doi: 10.1016/0042-6822(80)90560-7. [DOI] [PubMed] [Google Scholar]
- Ou J. H., Rice C. M., Dalgarno L., Strauss E. G., Strauss J. H. Sequence studies of several alphavirus genomic RNAs in the region containing the start of the subgenomic RNA. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5235–5239. doi: 10.1073/pnas.79.17.5235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papkoff J., Hunter T., Beemon K. In vitro translation of virion RNA from Moloney murine sarcoma virus. Virology. 1980 Feb;101(1):91–103. doi: 10.1016/0042-6822(80)90486-9. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Rottier P. J., Horzinek M. C., van der Zeijst B. A. Viral protein synthesis in mouse hepatitis virus strain A59-infected cells: effect of tunicamycin. J Virol. 1981 Nov;40(2):350–357. doi: 10.1128/jvi.40.2.350-357.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottier P. J., Spaan W. J., Horzinek M. C., van der Zeijst B. A. Translation of three mouse hepatitis virus strain A59 subgenomic RNAs in Xenopus laevis oocytes. J Virol. 1981 Apr;38(1):20–26. doi: 10.1128/jvi.38.1.20-26.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini D. D., Kreibich G., Morimoto T., Adesnik M. Mechanisms for the incorporation of proteins in membranes and organelles. J Cell Biol. 1982 Jan;92(1):1–22. doi: 10.1083/jcb.92.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schochetman G., Stevens R. H., Simpson R. W. Presence of infectious polyadenylated RNA in coronavirus avian bronchitis virus. Virology. 1977 Apr;77(2):772–782. doi: 10.1016/0042-6822(77)90498-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefton B. M., Beemon K., Hunter T. Comparison of the expression of the src gene of Rous sarcoma virus in vitro and in vivo. J Virol. 1978 Dec;28(3):957–971. doi: 10.1128/jvi.28.3.957-971.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddell S. G., Wege H., Barthel A., ter Meulen V. Coronavirus JHM: cell-free synthesis of structural protein p60. J Virol. 1980 Jan;33(1):10–17. doi: 10.1128/jvi.33.1.10-17.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddell S. Coronavirus JHM: coding assignments of subgenomic mRNAs. J Gen Virol. 1983 Jan;64(Pt 1):113–125. doi: 10.1099/0022-1317-64-1-113. [DOI] [PubMed] [Google Scholar]
- Siddell S., Wege H., Barthel A., ter Meulen V. Coronavirus JHM: intracellular protein synthesis. J Gen Virol. 1981 Mar;53(Pt 1):145–155. doi: 10.1099/0022-1317-53-1-145. [DOI] [PubMed] [Google Scholar]
- Siddell S., Wege H., Ter Meulen V. The biology of coronaviruses. J Gen Virol. 1983 Apr;64(Pt 4):761–776. doi: 10.1099/0022-1317-64-4-761. [DOI] [PubMed] [Google Scholar]
- Simmons D. T., Strauss J. H. Translation of Sindbis virus 26 S RNA and 49 S RNA in lysates of rabbit reticulocytes. J Mol Biol. 1974 Jun 25;86(2):397–409. doi: 10.1016/0022-2836(74)90027-8. [DOI] [PubMed] [Google Scholar]
- Spaan W. J., Rottier P. J., Horzinek M. C., van der Zeijst B. A. Isolation and identification of virus-specific mRNAs in cells infected with mouse hepatitis virus (MHV-A59). Virology. 1981 Jan 30;108(2):424–434. doi: 10.1016/0042-6822(81)90449-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaan W. J., Rottier P. J., Horzinek M. C., van der Zeijst B. A. Sequence relationships between the genome and the intracellular RNA species 1, 3, 6, and 7 of mouse hepatitis virus strain A59. J Virol. 1982 May;42(2):432–439. doi: 10.1128/jvi.42.2.432-439.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern D. F., Burgess L., Sefton B. M. Structural analysis of virion proteins of the avian coronavirus infectious bronchitis virus. J Virol. 1982 Apr;42(1):208–219. doi: 10.1128/jvi.42.1.208-219.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern D. F., Kennedy S. I. Coronavirus multiplication strategy. I. Identification and characterization of virus-specified RNA. J Virol. 1980 Jun;34(3):665–674. doi: 10.1128/jvi.34.3.665-674.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern D. F., Kennedy S. I. Coronavirus multiplication strategy. II. Mapping the avian infectious bronchitis virus intracellular RNA species to the genome. J Virol. 1980 Nov;36(2):440–449. doi: 10.1128/jvi.36.2.440-449.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern D. F., Sefton B. M. Coronavirus proteins: biogenesis of avian infectious bronchitis virus virion proteins. J Virol. 1982 Dec;44(3):794–803. doi: 10.1128/jvi.44.3.794-803.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern D. F., Sefton B. M. Coronavirus proteins: structure and function of the oligosaccharides of the avian infectious bronchitis virus glycoproteins. J Virol. 1982 Dec;44(3):804–812. doi: 10.1128/jvi.44.3.804-812.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern D. F., Sefton B. M. Synthesis of coronavirus mRNAs: kinetics of inactivation of infectious bronchitis virus RNA synthesis by UV light. J Virol. 1982 May;42(2):755–759. doi: 10.1128/jvi.42.2.755-759.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturman L. S., Holmes K. V., Behnke J. Isolation of coronavirus envelope glycoproteins and interaction with the viral nucleocapsid. J Virol. 1980 Jan;33(1):449–462. doi: 10.1128/jvi.33.1.449-462.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturman L. S., Holmes K. V. Characterization of coronavirus II. Glycoproteins of the viral envelope: tryptic peptide analysis. Virology. 1977 Apr;77(2):650–660. doi: 10.1016/0042-6822(77)90489-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturman L. S., Holmes K. V. The molecular biology of coronaviruses. Adv Virus Res. 1983;28:35–112. doi: 10.1016/S0065-3527(08)60721-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturman L. S. I. Structural proteins: effects of preparative conditions on the migration of protein in polyacrylamide gels. Virology. 1977 Apr;77(2):637–649. doi: 10.1016/0042-6822(77)90488-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadey C. N., Westaway E. G. Structural proteins and glycoproteins of infectious bronchitis virus particles labeled during growth in chick embryo cells. Intervirology. 1981;15(1):19–27. doi: 10.1159/000149210. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins H., Reeve P., Alexander D. J. The ribonucleic acid of infectious bronchitis virus. Arch Virol. 1975;47(3):279–286. doi: 10.1007/BF01317815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wege H., Siddell S., ter Meulen V. The biology and pathogenesis of coronaviruses. Curr Top Microbiol Immunol. 1982;99:165–200. doi: 10.1007/978-3-642-68528-6_5. [DOI] [PubMed] [Google Scholar]
- Wege H., Wege H., Nagashima K., ter Meulen V. Structural polypeptides of the murine coronavirus JHM. J Gen Virol. 1979 Jan;42(1):37–47. doi: 10.1099/0022-1317-42-1-37. [DOI] [PubMed] [Google Scholar]
- Weiss S. R., Leibowitz J. L. Comparison of the RNAs of murine and human coronaviruses. Adv Exp Med Biol. 1981;142:245–259. doi: 10.1007/978-1-4757-0456-3_20. [DOI] [PubMed] [Google Scholar]
- Wengler G., Wengler G. Localization of the 26-S RNA sequence on the viral genome type 42-S RNA isolated from SFV-infected cells. Virology. 1976 Aug;73(1):190–199. doi: 10.1016/0042-6822(76)90073-8. [DOI] [PubMed] [Google Scholar]