Abstract
The first committed step in the biosynthesis of l-ascorbate from d-glucose in plants requires conversion of GDP-l-galactose to l-galactose 1-phosphate by a previously unidentified enzyme. Here we show that the protein encoded by VTC2, a gene mutated in vitamin C-deficient Arabidopsis thaliana strains, is a member of the GalT/Apa1 branch of the histidine triad protein superfamily that catalyzes the conversion of GDP-l-galactose to l-galactose 1-phosphate in a reaction that consumes inorganic phosphate and produces GDP. In characterizing recombinant VTC2 from Arabidopsis thaliana as a specific GDP-l-galactose/GDP-d-glucose phosphorylase, we conclude that enzymes catalyzing each of the ten steps of the Smirnoff-Wheeler pathway from glucose to ascorbate have been identified. Finally, we identify VTC2 homologs in plants, invertebrates, and vertebrates, suggesting that a similar reaction is used widely in nature.
Vitamin C (l-ascorbic acid) is well known as an important antioxidant and enzyme cofactor in animals (1, 2) and in plants (3). Apparently, all plants are able to produce vitamin C and mutants completely deficient in synthesis have not been described, suggesting an essential role of ascorbate biosynthesis in these organisms (4). Though vertebrate vitamin C synthesis is restricted to one organ (liver in mammals, and kidney in fish, amphibians and reptiles) (5, 6), virtually all cells in plants can form ascorbate (4). In the few vertebrate species, such as humans, which lack ascorbate biosynthesis, loss of the pathway is compensated by dietary intake, particularly from plants.
Different pathways of ascorbate synthesis have evolved in animals and plants. In animals, ascorbate is formed from UDP-d-glucuronate in a pathway involving d-glucuronate formation, reduction and lactonization of d-glucuronate to l-gulonolactone and oxidation of the latter to l-ascorbate (reviewed in Ref. 7). Deficiency of the enzyme catalyzing this last step (l-gulonolactone oxidase) is responsible for the loss of ascorbate synthesis in the vitamin C-requiring vertebrates (8). In plants, the ascorbate synthesis pathway has remained elusive until recently and alternative pathways may exist (9). The Smirnoff-Wheeler pathway (10) has garnered strong biochemical and genetic support (11-16) and appears to represent the major route to ascorbate biosynthesis. In this pathway, GDP-d-mannose, formed from d-mannose 1-phosphate, is successively converted to GDP-l-galactose, l-galactose 1-phosphate, l-galactose, l-galactono-1,4-lactone, and finally to l-ascorbate.
Screens for ozone-sensitive (17) or ascorbate-deficient (18) mutants in Arabidopsis thaliana led to the identification of four loci (VTC1, VTC2, VTC3, and VTC4) involved in the maintenance of the vitamin C pool. Characterization of the vtc1 (19) and vtc4 (15) mutants, as well as biochemical studies (14), have allowed the identification of two of the enzymes required for l-ascorbic acid synthesis through the Smirnoff-Wheeler pathway. VTC1 and VTC4 encode GDP-mannose pyrophosphorylase (19) and l-Gal-1-P1 phosphatase (15), respectively. The function of the VTC2 and VTC3 genes has not yet been elucidated. The only step in the Smirnoff-Wheeler pathway that has not been identified with a gene product is the conversion of GDP-l-Gal to l-Gal-1-P.
Here we show that the product of the A. thaliana VTC2 gene, which has previously been cloned (20), is broadly conserved in plants and in animals. On the basis of sequence similarity and enzymatic analysis, we characterize VTC2 as a histidine triad (HIT) enzyme of the GalT/Apa1 branch possessing a high GDP-l-Gal phosphorylase activity, which produces GDP and the ascorbic acid precursor l-Gal-1-P. This finding completes the identification of the ten enzymatic steps from d-glucose to l-ascorbate in the Smirnoff-Wheeler pathway for vitamin C biosynthesis in plants.
EXPERIMENTAL PROCEDURES
Materials
ADP-d-Glc, GDP, GDP-d-Glc, GDP-d-Man, UDP-d-Gal, UDP-d-Glc, d-Gal-1-P and d-Man-1-P were from Sigma Chemical Co. (St. Louis, MO). All of the sugar nucleotides and sugar phosphates were in the α-configuration. l-Gal-1-P, in the β-configuration, was purchased from Glycoteam (Hamburg, Germany). All other reagents were of analytical grade. Recombinant A. thaliana GDP-d-mannose 3”,5”-epimerase (GME) containing an N-terminal His tag was prepared as described (21) from a construct provided by Prof. James H. Naismith (University of St. Andrews, UK). GDP-l-Gal was prepared by incubating 0.94 mM GDP-d-Man with GME (46.5 μg/ml) for 5.5 hours at 26 °C in 50 mM Tris-HCl, pH 7.5. After heating for 3 min at 98 °C, denatured GME was removed by centrifugation and the supernatant (containing 0.77 mM GDP-d-Man, 0.14 mM GDP-l-Gal, and 0.03 mM GDP-l-Gul) was used for determination of the kinetic constants of VTC2 for GDP-l-Gal.
Cloning, expression and purification of A. thaliana VTC2
The A. thaliana VTC2 coding sequence was amplified by PCR from a cDNA clone (U17921) provided by the Arabidopis Biological Resource Center at Ohio State University. This clone contains the coding region of the At4g26850 locus on chromosome 4 (GenBank Accession number BT006589) and was prepared by the Salk Institute Genomic Analysis Laboratory (22). The forward and reverse primers were 5′-CACCATGTTGAAAATCAAAAGAG and 5′-TCACTGAAGGACAAGGCACTCGG. The PCR product was cloned into the Champion pET100/D-Topo vector (Invitrogen, Carlsbad, CA), resulting in a construct adding a 36 amino-acid N-terminal extension (MRGSHHHHHHGMASMTGGQQMGRDLYDDDDKDHPFT) to the VTC2 protein. The DNA sequence of the insert was confirmed. The plasmid was transformed into E. coli BL21 Star (DE3) cells and grown until the optical density at 600 nm reached 0.5-0.6. Protein overexpression was induced with 0.4 mM isopropyl β-d-1-thiogalactopyranoside. After overnight incubation with shaking at 18 °C, cells were harvested, washed, frozen, resuspended in a pH 7.9 buffer containing 20 mM Tris-HCl, 500 mM NaCl, 5 mM imidazole, 0.1 mM PMSF and 1 μg/ml leupeptin, and lysed by sonication. Clarified lysate was loaded onto an Ni2+-charged column (Novagen, San Diego, CA) and eluted using a gradient of the resuspension buffer containing 5 mM to 1 M imidazole. Peak VTC2 protein fractions were pooled and buffer was exchanged to 10 mM HEPES, pH 7.5, 100 mM NaCl, 1 mM DTT in a 10 kDa cut-off Amicon Ultra Centrifugal Filter Unit (Millipore, Billerica, MA). Protein concentration was determined using a Lowry assay after precipitation with trichloroacetic acid. Purified enzyme was stored at -80 °C in 10% glycerol.
Enzymatic assays
Phosphorylase activity of purified recombinant A. thaliana VTC2 was assayed by measuring GDP formation after incubation with various GDP-hexoses in a reaction mixture at pH 7.5 containing 50 mM Tris-HCl, 5 mM sodium phosphate, 2 mM MgCl2, 10 mM NaCl, and 1 mM DTT. Reactions (26 °C) were initiated with enzyme and stopped after 5 to 60 min by heating at 98 °C for 3 min. After removal of precipitated protein by centrifugation, supernatants were analyzed by anion-exchange HPLC on a Partisil SAX column (10 μm bead size, 4.6 × 250 mm; Alltech Associates, Deerfield, IL) using a Hewlett Packard Series II 1090 liquid chromatograph. A gradient of 0.01-0.5 M NH4H2PO4, pH 3.7, was used at a flow rate of 2 ml/min. Nucleotides were detected by absorbance at 254 nm using a reference wavelength of 450 nm. GMP, GDP-hexoses and GDP eluted at ∼ 13, 17 and 24 min, respectively. To assay the enzymatic activity in the reverse direction (hexose 1-phosphate + GDP → GDP-hexose + Pi), GDP-hexose concentration was measured by the anion-exchange HPLC method after incubation of VTC2 with various hexose 1-phosphates and 5 mM GDP as described above, except that sodium phosphate and MgCl2 were omitted. GDP and GDP-hexose concentrations were calculated by comparing the integrated peak areas with those of standard GDP or GDP-d-Man solutions.
Mass spectrometry
Matrix-assisted laser desorption/ionization mass spectrometry was performed on a Voyager-DE STR MALDI-TOF instrument. GDP-d-Man, GDP-l-Gal and GDP-l-Gul were purified by reverse-phase HPLC (21) using an Econosphere C-18 column (5 μm bead size, 4.6 × 250 mm; Alltech Associates, Deerfield, IL). Fractions (15 s) were collected, dried, and resuspended in a solution of 0.29 M 3-hydroxypicolinic acid with 9 μM GDP as an internal calibrant in acetonitrile/H2O/trifluoroacetic acid (50/50/0.1, v/v/v). The samples were analyzed in positive ion mode and the mass accuracy was ∼ 10-30 ppm.
RESULTS
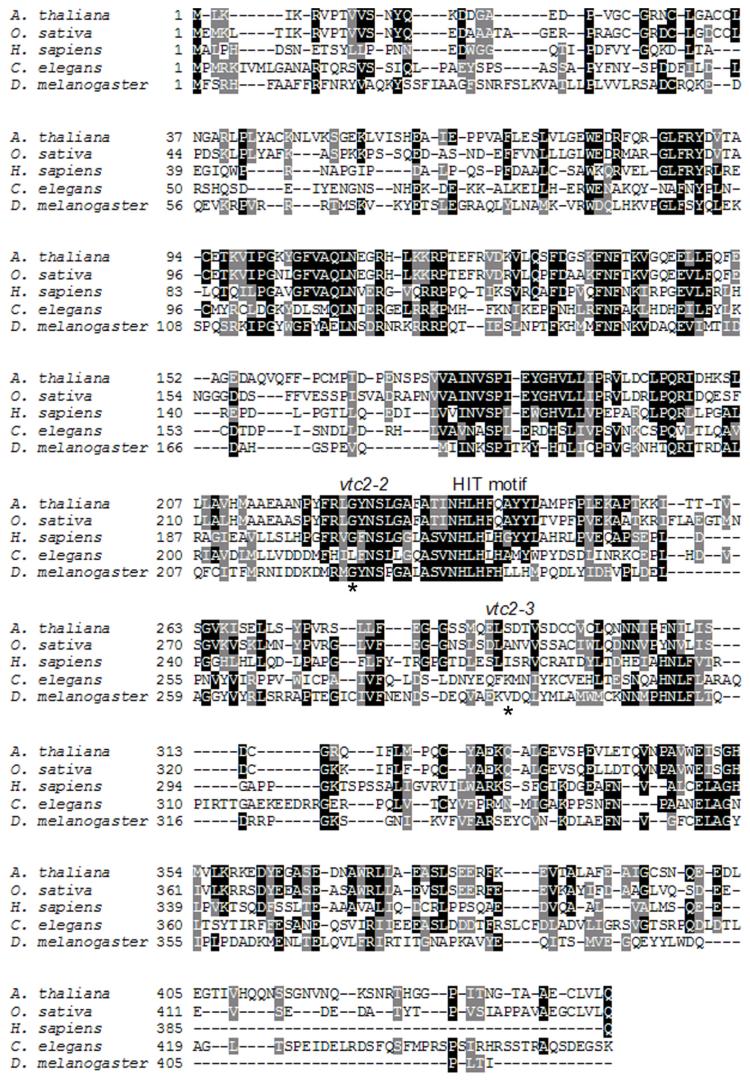
In our study of aging in the nematode worm Caenorhabditis elegans, we became interested in the C10F3.4 gene that partially overlaps the pcm-1 gene encoding the l-isoaspartyl protein methyltransferase (23). We examined the deduced amino acid sequence of C10F3.4 and, as shown in Fig. 1, found apparent orthologs in invertebrates, vertebrates and plants, including the VTC2 gene product of A. thaliana. By performing PSI-BLAST analyses (24) of the C10F3.4 and VTC2 gene products, we detected a low level of similarity with members of the HIT protein superfamily. HIT enzymes consist of nucleoside monophosphate hydrolases related to Hint, Fhit, Aprataxin and DcpS and nucleoside monophosphate transferases related to GalT, the d-galactose 1-phosphate uridylyltransferase and Apa1, a diadenosine tetraphosphate phosphorylase (reviewed in Ref. 25). In A. thaliana, the vtc2-2 and vtc2-3 missense mutations (20) map to either side of the HIT motif (Fig. 1). Since these mutants are vitamin C-deficient (18), we considered the possibility that a step in vitamin C biosynthesis is catalyzed by a HIT enzyme.
Fig. 1. Amino acid alignment of VTC2 homologs.
The A. thaliana VTC2 sequence is aligned with those of Oryza sativa, Homo sapiens, C. elegans and Drosophila melanogaster. Residues boxed in black are identical in three or more of the proteins. Residues boxed in grey are chemically similar. Asterisks mark the residues that are altered in the vtc2-2 and vtc2-3 A. thaliana mutant strains, namely G224D and S290F. The HIT motif is indicated. Accession numbers include BT006589 for A. thaliana, NP_001066338 for O. sativa, BAC85370 for H. sapiens, NP_001023638 for C. elegans, and NP_648319 for D. melanogaster.
HIT hydrolases and HIT transferases can be distinguished both by sequence and by enzymatic requirements. HIT hydrolases typically contain an active site motif including His-ϕ-His-ϕ-His, where ϕ is a hydrophobic amino acid. In contrast, the third His of the HIT motif is not conserved in HIT transferases and is frequently a Gln residue (25). As shown in Fig. 1, plant VTC2 sequences have a His-Leu-His-Phe-Gln sequence, whereas animal VTC2 homologs contain a His-Leu-His-(Leu/Phe)-His sequence. All known HIT enzymes use the second His residue of the HIT motif to attack the nucleoside monophosphate moiety of substrates, forming a covalent nucleotidylylated enzyme. In the case of HIT hydrolases, the nucleotidylylated intermediate is labile to water, such that it rapidly decomposes to enzyme plus nucleoside monophosphate. In contrast, the nucleotidylylated intermediate of HIT transferases such as GalT and Apa1 is stable to water and awaits reaction with phosphate (phosphorolysis) or a specific phosphorylated substrate (transfer). Given the lack of conservation of the third His residue in the HIT motif of the plant VTC2 sequence and the requirement of an intact VTC2 gene for maintenance of the ascorbate pool in A. thaliana, we hypothesized that VTC2 may be a GDP-l-galactose phosphorylase that would produce l-Gal-1-P plus GDP in a reaction requiring inorganic phosphate. Despite evidence for the activity, the enzyme that forms l-Gal-1-P remained unknown (11) and it has been widely assumed that such an enzyme would simply hydrolyze GDP-l-Gal into GMP plus l-Gal-1-P (10).
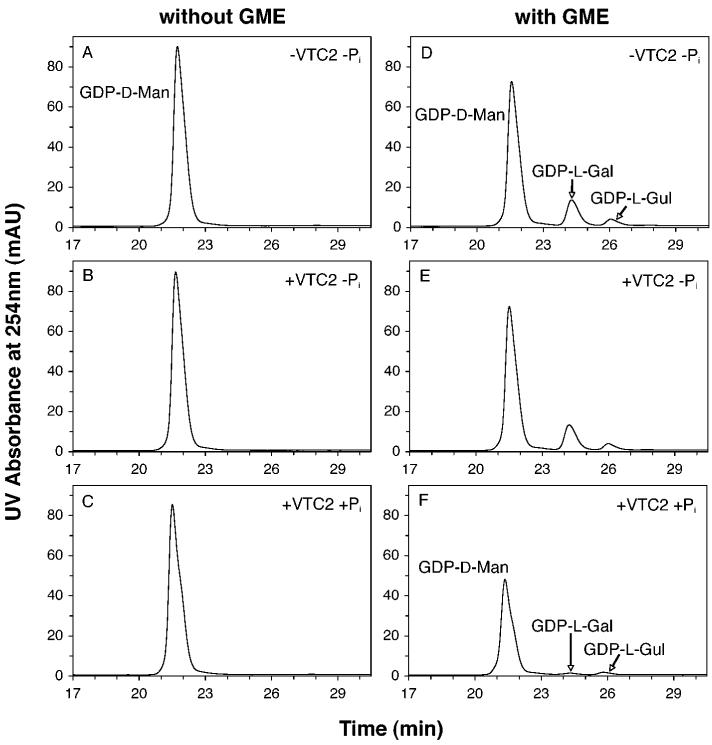
To test the hypothesis that VTC2 catalyzes the phosphorolysis of GDP-l-Gal, we purified recombinant A. thaliana VTC2 as an N-terminal His-tagged protein from a bacterial expression system. Because the GDP-l-Gal substrate was not commercially available, we prepared it enzymatically from GDP-d-Man using the recombinant GDP-d-mannose 3”,5”-epimerase (GME) from A. thaliana (21) in a coupled assay. We measured the consumption of GDP-d-Man in the presence of VTC2 without and with prior reaction with GME and without and with Pi. In the absence of GME, less than 2% of the initial GDP-d-Man was consumed after a 10-min incubation with VTC2, even when Pi was present in the reaction mixture (Fig. 2C). GME catalyzes the reversible epimerization of GDP-d-Man to form GDP-l-Gal and, to a lesser extent, GDP-l-gulose (GDP-l-Gul) (21, 26). The substrate and two products can be separated by reverse-phase HPLC (21). We confirmed this chromatographic separation with comparable elution times and an equilibrium product distribution of 82:14:3 for GDP-d-Man, GDP-l-Gal, and GDP-l-Gul (Fig. 2D), similar to that reported previously (80:15:5; Ref. 21). Furthermore, we confirmed the identification of the GME products by UV absorption spectrum (identical to that of standard GDP-d-Man), as well as by mass spectrometry with the detection of a positive ion of m/z 606, consistent with all three nucleotide sugars.
Fig. 2. Dependence of VTC2 activity on GME and Pi.
GDP-d-Man (A) is not a hydrolytic (B) nor phosphorolytic (C) substrate of VTC2. The equilibrium reaction products of GME activity on GDP-d-Man (D), namely GDP-d-Man, GDP-l-Gal and GDP-l-Gul are not hydrolytic substrates of VTC2 (E). However, VTC2 catalyzes a phosphate-dependent reaction that preferentially consumes GDP-l-Gal (F). In these experiments, samples were preincubated in the absence of VTC2 for 20 min at 26 °C in the presence of 0.14 mM GDP-d-Man with or without 23 μg/ml GME in a pH 7.5 buffer containing 50 mM Tris-HCl, 2 mM MgCl2, 10 mM NaCl, and 1 mM DTT. When indicated, sodium phosphate was added at a concentration of 5 mM. Incubations were then continued for 10 min at 26 °C with (B, C, E, F) or without (A, D) VTC2 at 6.5 μg/ml. The reactions were stopped by heating and 60 μl of the deproteinized reaction mixtures were adjusted to 100 mM NaCl and analyzed by reverse-phase HPLC as described by Major et al. (21) with the exception that an Econosphere C-18 column (5 μm bead size, 4.6 × 250 mm; Alltech Associates, Deerfield, IL) was used. The results shown are representative of at least 3 independent experiments. Elution times for GMP and GDP were 21 and 43 min, respectively.
Addition of VTC2 in the absence of Pi had no effect on the concentration of the GDP-hexoses present in the GME reaction mixture (Fig. 2E). However, in the presence of Pi, VTC2 addition led to a decrease of the three GDP-hexose peaks and, significantly, a modification of the ratio of these peaks (Fig. 2F). After a 10-min incubation with VTC2 and 5 mM Pi, GDP-d-Man, GDP-l-Gal and GDP-l-Gul were found in the ratio 97:1:2, indicating that VTC2 preferentially acts on GDP-l-Gal. The Pi dependence of this enzymatic reaction is consistent with the prediction from sequence analysis above that VTC2 is a phosphorylase rather than a hydrolase.
To confirm the activity of VTC2 with GDP-l-Gal, we incubated VTC2 in the presence of Pi with the GDP-hexose mixture formed by reaction of GME with GDP-d-Man, but under conditions where GME was inactivated by heating prior to the addition of VTC2. Under these conditions, VTC2 only affected the GDP-l-Gal concentration, which decreased linearly with incubation time (Figs. 3A-D). Parallel analysis of the reaction mixtures by anion-exchange HPLC clearly showed that the consumption of GDP-l-Gal was correlated with the appearance of a peak eluting at ∼ 24 min and identified as GDP by UV spectrum and coelution with standard (Figs. 3E-H). No formation of GMP was detected in these experiments and the production of GDP was precisely matched to the consumption of GDP-l-Gal.
Fig. 3. VTC2-catalyzed phosphorolysis of GDP-l-Gal and formation of GDP.
GDP-d-Man was preincubated for 30 min with GME and 5 mM sodium phosphate as described in Fig. 2. After removal of heat-inactivated (3 min at 98 °C) GME, GDP-hexose consumption and GDP formation were monitored after incubation for the indicated times with VTC2 (6.5 ng/ml). Deproteinized reaction mixtures were analyzed by reverse-phase HPLC (panels A—C) as described in Fig. 2 and anion-exchange HPLC (100 μl samples, panels E—G) as described in the “Experimental Procedures” section. A small amount of GDP (less than 1% of the total GDP-hexose pool) was detected at 0 min as a VTC2-independent hydrolysis product. The HPLC traces shown are representative of three separate experiments. In panels D and H, the concentrations of GDP-d-Man, GDP-l-Gal, GDP-l-Gul and GDP are given as means ± standard deviations calculated from these experiments. Only error bars exceeding the symbol sizes are represented.
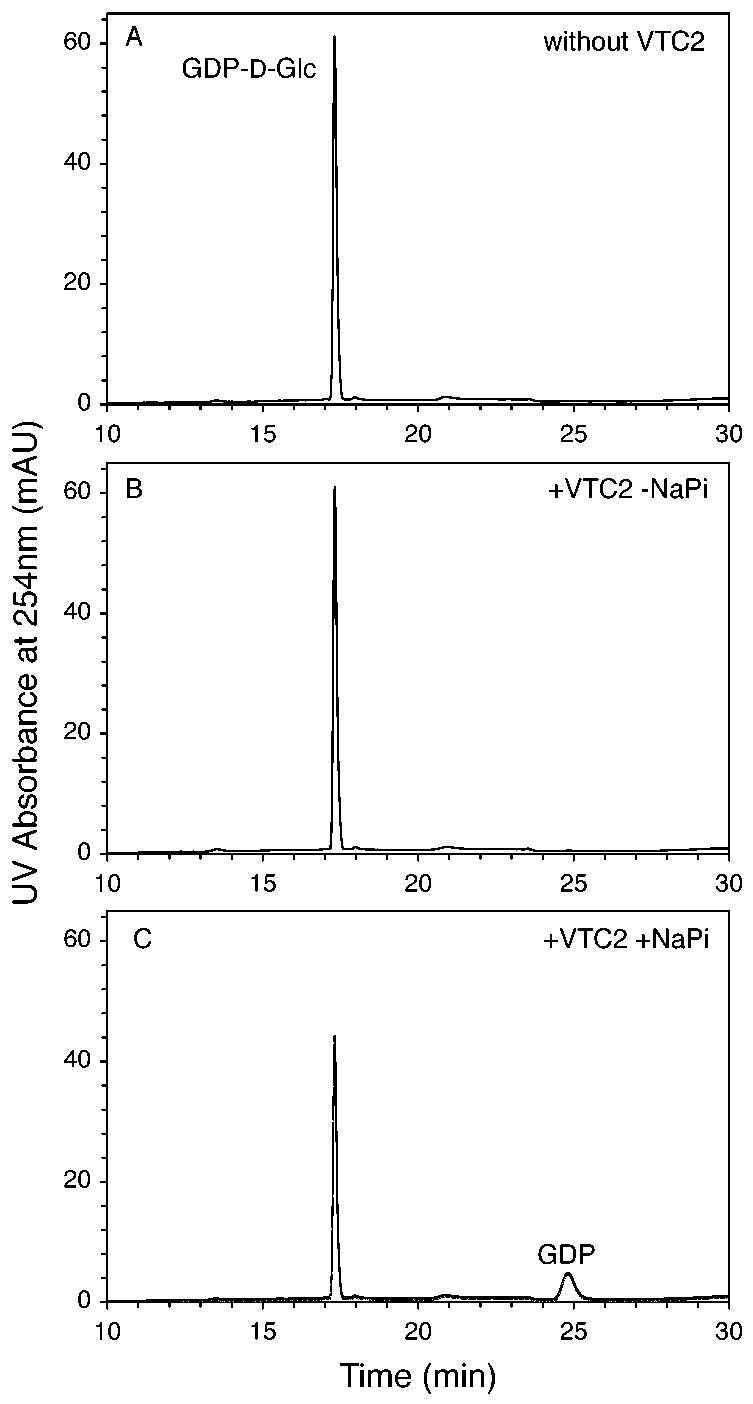
To characterize the substrate specificity of VTC2, we incubated several nucleoside diphosphate hexoses at a concentration of 1 mM for 10 min (26 °C) in the presence of Pi with or without VTC2 (32.5 μg/ml). NDP-sugar consumption (and NDP formation) were measured by anion-exchange HPLC. No phosphorylase activity was detected when UDP-d-Glc, UDP-d-Gal or ADP-d-Glc were used as substrates and only a very low activity was found in the presence of GDP-d-Man. However, an almost total conversion of the NDP-sugar to NDP was observed with GDP-d-Glc in the incubation conditions used (data not shown).
Kinetic analysis of the VTC2 phosphorylase activity confirmed that GDP-d-Glc is a good substrate for VTC2 (Table 1). A mixture obtained by GME incubation with GDP-d-Man (and subsequent elimination of GME) was used to determine the kinetic properties of VTC2 for the phosphorolysis of GDP-l-Gal, under conditions where GDP formation reflects only this reaction. VTC2 also exhibited a low Km value and a high turnover rate with GDP-l-Gal, the values obtained resembling closely those measured with GDP-d-Glc. GDP-d-Man, on the other hand, is a poorer substrate for VTC2 by a factor of ∼ 30,000 in the kcat/Km term (Table 1).
TABLE 1. Substrate specificity of the A. thaliana VTC2 phosphorylase.
Km and Vmax values were obtained by fitting the experimental data to the Michaelis-Menten equation usingthe Km calculator of the BioMechanic program (http://www.biomechanic.org/). Enzymatic turnover numbers were derived from the Vmax values by using a molecular weight of 53.1 kDa for recombinant His-tagged VTC2 with the assumption that the enzyme preparation is pure. Incubation times and enzyme concentrations were adjusted to obtain initial velocity data. Values are means ± standard deviation calculated from at least 3 individual experiments for each substrate. Enzymatic activities were measured as described in the “Experimental Procedures” section and substrate concentrations ranged from 3.4-54 μM (GDP-l-Gal), 2.5-100 μM (GDP-d-Glc), 0.25-5 mM (GDP-d-Man), and 2.5-40 mM (hexose 1-phosphates)
| A. Forward reaction | |||
|---|---|---|---|
| Substrate | kcat (s-1) | Km (mM) | kcat/Km (s-1 M-1) |
| GDP-l-Gal | 64 ± 8 | 0.010 ± 0.001 | 6.3 ± 0.9 ×106 |
| GDP-d-Glc | 23 ± 3 | 0.0044 ± 0.0016 | 5.7 ± 2.3 ×106 |
| GDP-d-Man | 0.093 ± 0.011 | 0.52 ± 0.15 | 1.9 ± 0.3 ×102 |
| B. Reverse reaction | |||
|---|---|---|---|
| Substrate | kcat (s-1) | Km (mM) | kcat/Km (s-1 M-1) |
| l-Gal-1-P | 6.0 ± 0.4 | 45 ± 7 | 133 ± 12 |
| d-Glc-1-P | 12 ± 0.5 | 29 ± 4 | 412 ± 50 |
| d-Man-1-P | 0.0013 ± 0.0001 | 54 ± 8 | 0.025 ± 0.001 |
| d-Gal-1-P | 0.023 ± 0.002 | 67 ± 3 | 0.34 ± 0.02 |
VTC2 activity could also be measured in the reverse direction by incubating the enzyme in the presence of high concentrations of GDP and various hexose 1-phosphates, and assaying GDP-hexose formation in the absence of added Pi. Table 1 shows that the Km values of VTC2 for l-Gal-1-P and d-Glc-1-P are more than 3 orders of magnitude higher than for the corresponding GDP-sugar derivatives. The kcat terms for the reverse reaction with these hexose 1-phosphates are, however, relatively high (2- to 10-fold lower than those found in the direct reaction) and exceed by 2 and 3 orders of magnitude those measured with d-Gal-1-P and d-Man-1-P, respectively. Thus, VTC2 functions much better in the reverse reaction with l-Gal-1-P or d-Glc-1-P than with d-Man-1-P or d-Gal-1-P, confirming the forward reaction products and the specificity.
DISCUSSION
Our demonstration that VTC2 catalyzes the conversion of GDP-l-Gal to l-Gal-1-P completes the identification of genes encoding the enzymes of the Smirnoff-Wheeler pathway of ascorbate synthesis in plants (Fig. 4). We characterize VTC2 as a GDP-l-Gal/GDP-d-Glc phosphorylase. The phosphate requirement of VTC2 and its production of GDP are consistent with the presence of a Gln residue at position 5 of the HIT motif (25). Two well-characterized HIT transferases are GalT and Apa1. GalT reacts with UDP-d-Glc to produce a uridylylated enzyme intermediate plus d-Glc-1-P. The uridylylated enzyme is then intercepted by d-Gal-1-P to produce UDP-d-Gal (27). In the case of Apa1, the substrate is diadenosine tetraphosphate, which reacts to form adenylylated enzyme plus ATP. The adenylylated intermediate, stable to water, is phosphorolyzed by Pi to produce an ADP product (28). It can thus be predicted that VTC2, in the presence of GDP-l-Gal or GDP-d-Glc, forms a guanylylated enzyme intermediate at His238, which is phosphorolyzed by Pi to yield free enzyme and GDP.
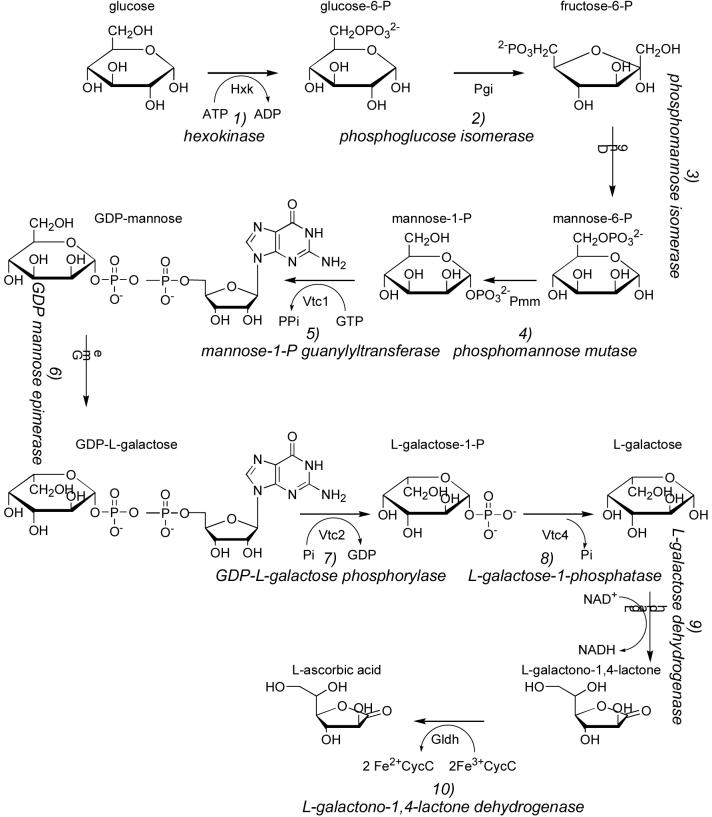
Fig. 4. Smirnoff-Wheeler pathway from d-glucose to l-ascorbic acid.
The forward, on-pathway reactions are depicted, with sugars in the d-configuration unless otherwise designated. The pathway begins with six steps used to produce GDP-Man and GDP-l-Gal for protein glycosylation and/or cell wall biosynthesis. In the seventh and committing step for l-ascorbic acid synthesis, the GDP-l-Gal product of GME is recognized by VTC2, the GDP-l-Gal phosphorylase, to produce l-Gal-1-P. In the eighth step, l-Gal is generated by VTC4, the l-Gal-1-P phosphatase. l-Gal is then oxidized to l-galactono-1,4-lactone and l-ascorbic acid by the NAD+-dependent and ferric cytochrome c-dependent enzymes, l-galactose dehydrogenase and l-galactono-1,4-lactone dehydrogenase.
As shown in Fig. 4, conversion of d-Glc to l-ascorbic acid through the Smirnoff-Wheeler pathway takes a remarkable 10 steps, 8 of which are required simply to convert d-Glc to l-Gal. l-Gal, representing the epimerization product of d-Gal at four carbon atoms, is rarely found in nature (29), emphasizing the importance of this portion of the pathway. Because two of the interconverted substrates and products of GME, namely GDP-d-Man and GDP-l-Gal, are utilized in biosynthesis of cell wall polysaccharides (30, 31) and/or protein glycosylation (32), the first committed step in the production of ascorbic acid is catalyzed by VTC2. Thus, VTC2 activity may be regulated to coordinate cellular needs for ascorbate with needs for cell wall synthesis and protein glycosylation.
In the Leloir pathway of galactose utilization, cells evolved a pathway to convert d-Gal to d-Glc equivalents by coupling a HIT family transferase (GalT) with an epimerase (UDP-d-galactose 4-epimerase) (27). Similar enzymology is used in the Smirnoff-Wheeler pathway, in which an epimerase (GME) is coupled to a HIT family phosphorylase (VTC2) to convert d-Man to l-Gal. In the former case, a specialty sugar is converted to a commodity sugar. The Leloir pathway moves forward by supplying d-Glc as UDP-d-Glc, using GalT to convert d-Gal-1-P plus UDP-d-Glc to d-Glc-1-P plus UDP-d-Gal, and epimerizing UDP-d-Gal to UDP-d-Glc. In the Smirnoff-Wheeler pathway, a commodity sugar is converted to a specialty sugar by virtue of epimerization prior to substrate selection by the HIT enzyme (Fig. 4).
VTC2 exhibits striking substrate specificity for GDP-l-Gal over GDP-d-Man and no apparent activity on GDP-l-Gul. This high selectivity of VTC2 for one of the GME products pulls the equilibrium of the GME-catalyzed reversible reaction in favor of GDP-d-Man conversion to GDP-l-Gal, which is made irreversible in the cell by the activity of the specific l-Gal-1-P phosphatase, VTC4 (14, 15). The specificity of these enzymes suggests that the main flux of ascorbate biosynthesis passes through the l-galactose branch, represented in Fig. 4, and not a putative l-gulose branch through formation of l-gulono-1,4-lactone, the vitamin C precursor in animals. The l-gulose branch has been suggested as a possible alternative ascorbate biosynthesis pathway (26), but enzymes for it have not been identified in plants (9).
While VTC2 exhibits strong discrimination against the GME equilibrium products, GDP-d-Man and GDP-l-Gul, and against ADP-d-Glc, UDP-d-Gal and UDP-d-Glc, we were surprised at the lack of discrimination between GDP-l-Gal and GDP-d-Glc. In the forward direction, both substrates are converted to hexose 1-phosphates with a specificity constant of ∼ 6 × 106 s-1 M-1. In the reverse direction, while the enzyme is nearly inert at converting d-Man-1-P, the C2 epimer of d-Glc-1-P, or the d-form of Gal-1-P, the enzyme has robust activity on d-Glc-1-P. In fact, the enzyme is three times more efficient at forming GDP-d-Glc than GDP-l-Gal. There are two potential explanations for the lack of specificity. First, although GDP-d-Glc is apparently formed in plants (33) where it can be used for the synthesis of cell wall polysaccharides (34), this metabolite may be spatially separated from VTC2, such that no evolutionary selection against d-Glc-1-P formation has occurred. In this regard it will be important to determine whether plant GDP-Man pyrophosphorylase (VTC1), which is responsible for GDP-d-Man formation in the Smirnoff-Wheeler pathway, discriminates against GDP-d-Glc formation as reported for the mammalian ortholog of VTC1, GDP-Man pyrophosphorylase B (35). Alternatively, if VTC1 does not discriminate against d-Glc-1-P and significant GDP-d-Glc is formed, the activity of VTC2 on this metabolite could serve to recycle d-Glc-1-P such that there is a second chance to make the fructose and mannose phosphates required for ascorbate synthesis.
Of the four Arabidopsis genes whose mutations result in vitamin C deficiency, the roles of three have now been established. The function of VTC3 remains unclear. Vitamin C turnover is apparently not affected in the vtc3 mutant (11) suggesting that VTC3 is not involved in ascorbate catabolism. Furthermore, the conversion of d-[U-14C]Man to [14C]ascorbic acid has been reported to be decreased in the vtc3 mutant (11). Thus, VTC3 may encode a protein involved in the regulation of the Smirnoff-Wheeler pathway or participating in an alternative ascorbate biosynthesis pathway.
Finally, what can we say about the role of the C10F3.4 gene product of C. elegans that initiated our interest in this project? While we detect small amounts of vitamin C in extracts of these worms (data not shown), we do not find orthologs of other distinctive enzymes of the Smirnoff-Wheeler pathway. The presence of a VTC2 homolog in humans, in which a role in vitamin C synthesis is unlikely, suggests that a different function is conserved in animals, potentially in glucose metabolism. Ongoing work is designed to further dissect the function of Arabidopsis VTC2 and to determine the functions of the apparent animal orthologs.
Acknowledgments
We thank Prof. James H. Naismith for providing the GME clone and the Arabidopsis Biological Resource Center at Ohio State University for providing the VTC2 clone. We are indebted to Kristofor Webb for assistance with mass spectrometry. Finally, we are grateful to Prof. Emile Van Schaftingen for critical reading of the manuscript.
Footnotes
This work was supported by National Institutes of Health grants GM026020 to S. G. C. (with a supplement for T. A. G.), AG018000 to S. G. C., and CA75954 to C. B. and by a National Science Foundation grant MCB-0448533 to S. G. C.. C. L. L. was supported by a research fellowship (bourse de formation-recherche) from the Luxembourgish Government.
- Gal-1-P
- galactose 1-phosphate
- GDP-l-Gul
- GDP-l-gulose
- Glc-1-P
- glucose 1-phosphate
- GME
- GDP-d-mannose 3”,5”-epimerase
- HIT
- histidine triad
- Man-1-P
- mannose 1-phosphate.
REFERENCES
- 1.Padayatty SJ, Katz A, Wang Y, Eck P, Kwon O, Lee JH, Chen S, Corpe C, Dutta A, Dutta SK, Levine M. J. Am. Coll. Nutr. 2003;22:18–35. doi: 10.1080/07315724.2003.10719272. [DOI] [PubMed] [Google Scholar]
- 2.Englard S, Seifter S. Annu. Rev. Nutr. 1986;6:365–406. doi: 10.1146/annurev.nu.06.070186.002053. [DOI] [PubMed] [Google Scholar]
- 3.Smirnoff N. Curr. Opin. Plant Biol. 2000;3:229–235. [PubMed] [Google Scholar]
- 4.De Tullio MC, Arrigoni O. Cell Mol. Life Sci. 2004;61:209–219. doi: 10.1007/s00018-003-3203-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chatterjee IB. Science. 1973;182:1271–1272. doi: 10.1126/science.182.4118.1271. [DOI] [PubMed] [Google Scholar]
- 6.Moreau R, Dabrowski K. Proc. Natl. Acad. Sci. U. S. A. 1998;95:10279–10282. doi: 10.1073/pnas.95.17.10279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Linster CL, Van Schaftingen E. FEBS J. 2007;274:1–22. doi: 10.1111/j.1742-4658.2006.05607.x. [DOI] [PubMed] [Google Scholar]
- 8.Nishikimi M, Fukuyama R, Minoshima S, Shimizu N, Yagi K. J. Biol. Chem. 1994;269:13685–13688. [PubMed] [Google Scholar]
- 9.Valpuesta V, Botella MA. Trends Plant Sci. 2004;9:573–577. doi: 10.1016/j.tplants.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Wheeler GL, Jones MA, Smirnoff N. Nature. 1998;393:365–369. doi: 10.1038/30728. [DOI] [PubMed] [Google Scholar]
- 11.Smirnoff N, Conklin PL, Loewus FA. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001;52:437–467. doi: 10.1146/annurev.arplant.52.1.437. [DOI] [PubMed] [Google Scholar]
- 12.Wolucka BA, Persiau G, Van Doorsselaere J, Davey MW, Demol H, Vandekerckhove J, Van Montagu M, Zabeau M, Boerjan W. Proc. Natl. Acad. Sci. U. S. A. 2001;98:14843–14848. doi: 10.1073/pnas.011578198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gatzek S, Wheeler GL, Smirnoff N. Plant J. 2002;30:541–553. doi: 10.1046/j.1365-313x.2002.01315.x. [DOI] [PubMed] [Google Scholar]
- 14.Laing WA, Bulley S, Wright M, Cooney J, Jensen D, Barraclough D, MacRae E. Proc. Natl. Acad. Sci. U. S. A. 2004;101:16976–16981. doi: 10.1073/pnas.0407453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conklin PL, Gatzek S, Wheeler GL, Dowdle J, Raymond MJ, Rolinski S, Isupov M, Littlechild JA, Smirnoff N. J. Biol. Chem. 2006;281:15662–15670. doi: 10.1074/jbc.M601409200. [DOI] [PubMed] [Google Scholar]
- 16.Qian W, Yu C, Qin H, Liu X, Zhang A, Johansen IE, Wang D. Plant J. 2007;49:399–413. doi: 10.1111/j.1365-313X.2006.02967.x. [DOI] [PubMed] [Google Scholar]
- 17.Conklin PL, Williams EH, Last RL. Proc. Natl. Acad. Sci. U. S. A. 1996;93:9970–9974. doi: 10.1073/pnas.93.18.9970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conklin PL, Saracco SA, Norris SR, Last RL. Genetics. 2000;154:847–856. doi: 10.1093/genetics/154.2.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Conklin PL, Norris SR, Wheeler GL, Williams EH, Smirnoff N, Last RL. Proc. Natl. Acad. Sci. U. S. A. 1999;96:4198–4203. doi: 10.1073/pnas.96.7.4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jander G, Norris SR, Rounsley SD, Bush DF, Levin IM, Last RL. Plant Physiol. 2002;129:440–450. doi: 10.1104/pp.003533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Major LL, Wolucka BA, Naismith JH. J. Am. Chem. Soc. 2005;127:18309–18320. doi: 10.1021/ja056490i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamada K, Lim J, Dale JM, Chen H, Shinn P, Palm CJ, Southwick AM, Wu HC, Kim C, Nguyen M, Pham P, Cheuk R, Karlin-Newmann G, Liu SX, Lam B, Sakano H, Wu T, Yu G, Miranda M, Quach HL, Tripp M, Chang CH, Lee JM, Toriumi M, Chan MM, Tang CC, Onodera CS, Deng JM, Akiyama K, Ansari Y, Arakawa T, Banh J, Banno F, Bowser L, Brooks S, Carninci P, Chao Q, Choy N, Enju A, Goldsmith AD, Gurjal M, Hansen NF, Hayashizaki Y, Johnson-Hopson C, Hsuan VW, Iida K, Karnes M, Khan S, Koesema E, Ishida J, Jiang PX, Jones T, Kawai J, Kamiya A, Meyers C, Nakajima M, Narusaka M, Seki M, Sakurai T, Satou M, Tamse R, Vaysberg M, Wallender EK, Wong C, Yamamura Y, Yuan S, Shinozaki K, Davis RW, Theologis A, Ecker JR. Science. 2003;302:842–846. doi: 10.1126/science.1088305. [DOI] [PubMed] [Google Scholar]
- 23.Kagan RM, Clarke S. Biochemistry. 1995;34:10794–10806. doi: 10.1021/bi00034a012. [DOI] [PubMed] [Google Scholar]
- 24.Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brenner C. Biochemistry. 2002;41:9003–9014. doi: 10.1021/bi025942q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolucka BA, Van Montagu M. J. Biol. Chem. 2003;278:47483–47490. doi: 10.1074/jbc.M309135200. [DOI] [PubMed] [Google Scholar]
- 27.Frey PA. FASEB J. 1996;10:461–470. [PubMed] [Google Scholar]
- 28.Plateau P, Fromant M, Schmitter J-M, Blanquet S. J. Bacteriol. 1990;172:6892–6899. doi: 10.1128/jb.172.12.6892-6899.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leang K, Maekawa K, Menavuvu BT, Morimoto K, Granstrom TB, Takada G, Izumori K. J. Biosci. Bioeng. 2004;97:383–388. doi: 10.1016/S1389-1723(04)70223-6. [DOI] [PubMed] [Google Scholar]
- 30.Roberts RM. Arch. Biochem. Biophys. 1971;145:685–692. doi: 10.1016/s0003-9861(71)80029-2. [DOI] [PubMed] [Google Scholar]
- 31.Baydoun EAH, Fry SC. J. Plant Physiol. 1988;132:484–490. [Google Scholar]
- 32.Rayon C, Cabanes-Macheteau M, Loutelier-Bourhis C, Salliot-Maire I, Lemoine J, Reiter WD, Lerouge P, Faye L. Plant Physiol. 1999;119:725–733. doi: 10.1104/pp.119.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barber GA, Hassid WZ. Biochim. Biophys. Acta. 1964;86:397–399. doi: 10.1016/0304-4165(64)90069-8. [DOI] [PubMed] [Google Scholar]
- 34.Piro G, Zuppa A, Dalessandro G, Northcote DH. Planta. 1993;190:206–220. doi: 10.1007/BF00196613. [DOI] [PubMed] [Google Scholar]
- 35.Ning B, Elbein AD. Eur. J. Biochem. 2000;267:6866–6874. doi: 10.1046/j.1432-1033.2000.01781.x. [DOI] [PubMed] [Google Scholar]