Abstract
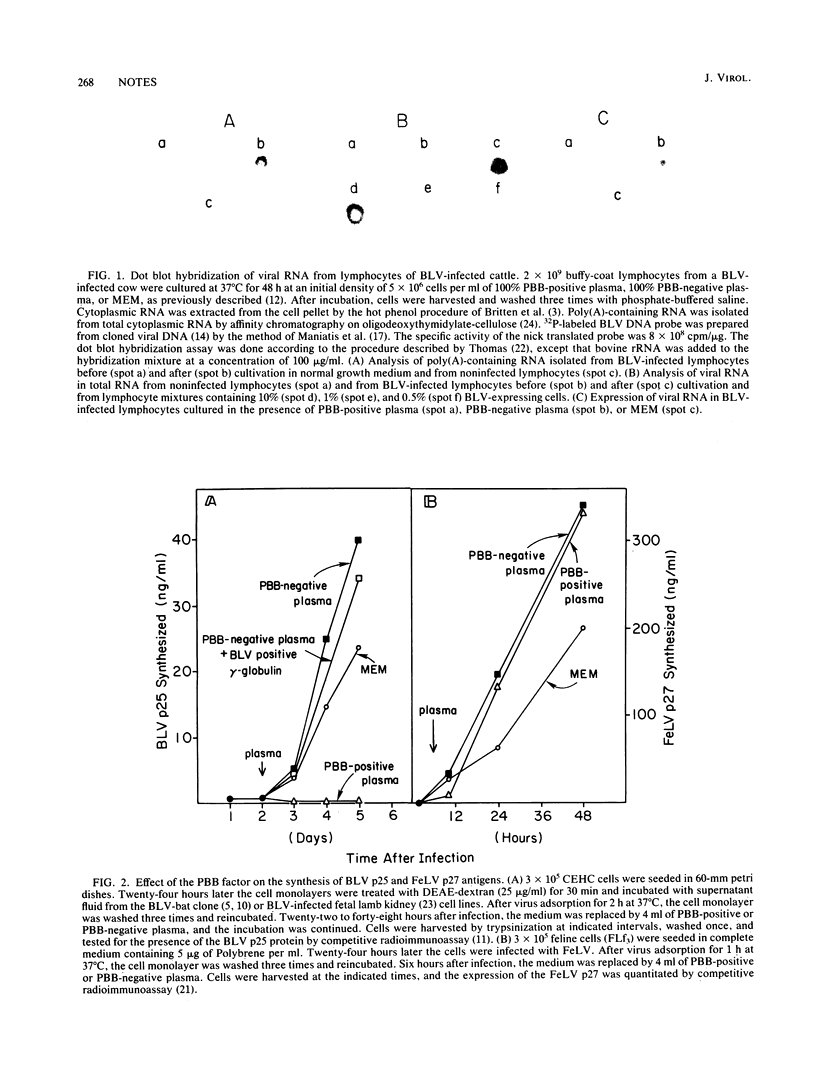
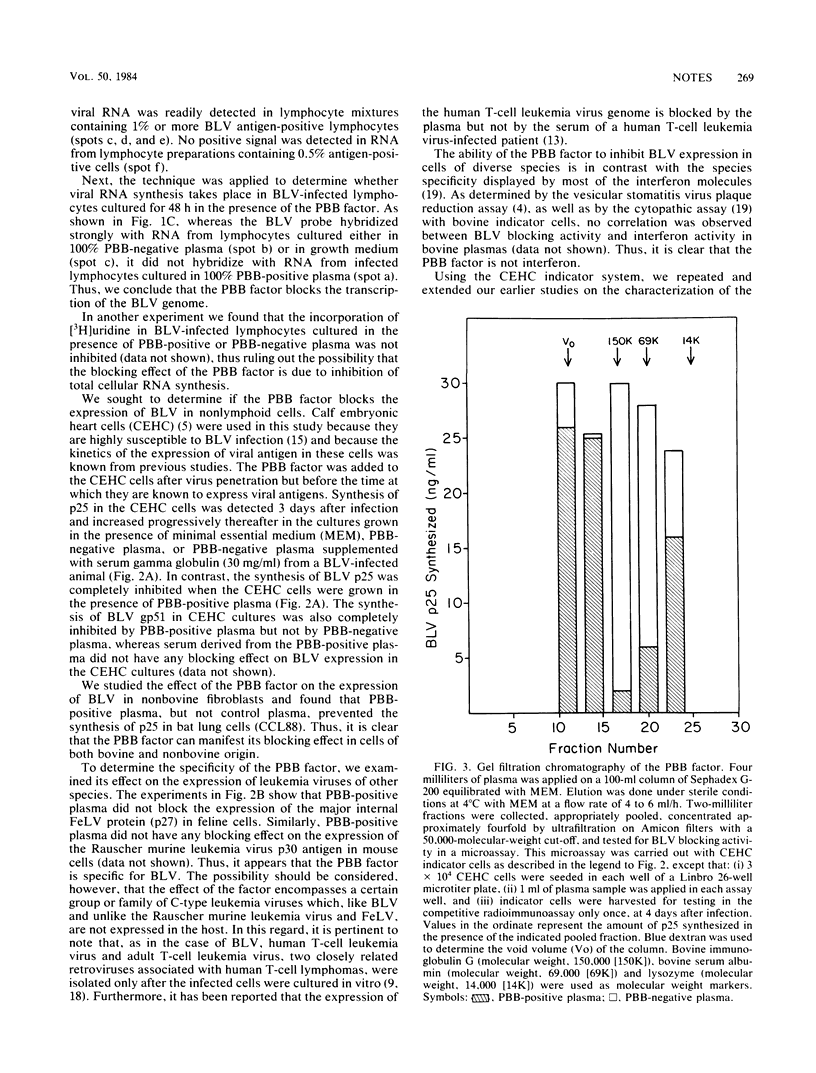
Using cloned bovine leukemia virus (BLV) DNA as a probe in the dot blot hybridization technique, we demonstrated that the expression of the BLV genome in infected lymphocytes is blocked in vivo at the transcriptional level. This blocking effect is due to a non-immunoglobulin protein present in the plasma but not in the serum of BLV-infected cattle. The plasma BLV-blocking protein also blocks the expression of the BLV genome in fibroblast cells of bovine and nonbovine origin infected with BLV in vitro. The plasma BLV-blocking factor has no inhibitory effect on the expression of Rauscher murine leukemia virus and feline leukemia virus in monolayer culture. The plasma BLV-blocking factor is not an interferon molecule. As determined by gel filtration chromatography, the plasma BLV-blocking factor has an apparent molecular weight of ca. 150,000.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baliga V., Ferrer J. F. Expression of the bovine leukemia virus and its internal antigen in blood lymphocytes. Proc Soc Exp Biol Med. 1977 Nov;156(2):388–391. doi: 10.3181/00379727-156-39942. [DOI] [PubMed] [Google Scholar]
- Baron S., McKerlie L. Broadly active inhibitor of viruses spontaneously produced by many cell types in culture. Infect Immun. 1981 May;32(2):449–453. doi: 10.1128/iai.32.2.449-453.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britten R. J., Graham D. E., Neufeld B. R. Analysis of repeating DNA sequences by reassociation. Methods Enzymol. 1974;29:363–418. doi: 10.1016/0076-6879(74)29033-5. [DOI] [PubMed] [Google Scholar]
- Chatterjee S., Hunter E. Inhibition of Mason-Pfizer monkey virus-induced syncytium formation in normal human cells by homologous interferon. Virology. 1980 Jul 30;104(2):487–490. doi: 10.1016/0042-6822(80)90351-7. [DOI] [PubMed] [Google Scholar]
- Diglio C. A., Ferrer J. F. Induction of syncytia by the bovine C-type leukemia virus. Cancer Res. 1976 Mar;36(3):1056–1067. [PubMed] [Google Scholar]
- Ferrer J. F., Avila L., Stock N. D. Serological detection of type C viruses found in bovine cultures. Cancer Res. 1972 Sep;32(9):1864–1870. [PubMed] [Google Scholar]
- Ferrer J. F. Bovine lymphosarcoma. Adv Vet Sci Comp Med. 1980;24:1–68. [PubMed] [Google Scholar]
- Gotoh Y. I., Sugamura K., Hinuma Y. Healthy carriers of a human retrovirus, adult T-cell leukemia virus (ATLV): demonstration by clonal culture of ATLV-carrying T cells from peripheral blood. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4780–4782. doi: 10.1073/pnas.79.15.4780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves D. C., Ferrer J. F. In vitro transmission and propagation of the bovine leukemia virus in monolayer cell cultures. Cancer Res. 1976 Nov;36(11 Pt 1):4152–4159. [PubMed] [Google Scholar]
- Gupta P., Ferrer J. F. Comparison of various serological and direct methods for the diagnosis of BLV infection in cattle. Int J Cancer. 1981 Aug 15;28(2):179–184. doi: 10.1002/ijc.2910280211. [DOI] [PubMed] [Google Scholar]
- Gupta P., Ferrer J. F. Expression of bovine leukemia virus genome is blocked by a nonimmunoglobulin protein in plasma from infected cattle. Science. 1982 Jan 22;215(4531):405–407. doi: 10.1126/science.6276975. [DOI] [PubMed] [Google Scholar]
- Haynes B. F., Miller S. E., Palker T. J., Moore J. O., Dunn P. H., Bolognesi D. P., Metzgar R. S. Identification of human T cell leukemia virus in a Japanese patient with adult T cell leukemia and cutaneous lymphomatous vasculitis. Proc Natl Acad Sci U S A. 1983 Apr;80(7):2054–2058. doi: 10.1073/pnas.80.7.2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerabek L., Gupta P., Ferrer J. F. An infectivity assay for bovine leukemia virus using the immunoperoxidase technique. Cancer Res. 1979 Oct;39(10):3952–3954. [PubMed] [Google Scholar]
- Kashmiri S. V., Mehdi R., Ferrer J. F. Molecular cloning of covalently closed circular DNA of bovine leukemia virus. J Virol. 1984 Feb;49(2):583–587. doi: 10.1128/jvi.49.2.583-587.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettmann R., Marbaix G., Cleuter Y., Portetelle D., Mammerickx M., Burny A. Genomic integration of bovine leukemia provirus and lack of viral RNA expression in the target cells of cattle with different responses to BLV infection. Leuk Res. 1980;4(6):509–519. doi: 10.1016/0145-2126(80)90062-4. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poiesz B. J., Ruscetti F. W., Gazdar A. F., Bunn P. A., Minna J. D., Gallo R. C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock N. D., Ferrer J. F. Replicating C-type virus in phytohemagglutinin-treated buffy-coat cultures of bovine origin. J Natl Cancer Inst. 1972 Apr;48(4):985–996. [PubMed] [Google Scholar]
- Strand M., August J. T. Structural proteins of mammalian oncogenic RNA viruses: multiple antigenic determinants of the major internal protein and envelope glycoprotein. J Virol. 1974 Jan;13(1):171–180. doi: 10.1128/jvi.13.1.171-180.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. Y., Hayward W. S., Hanafusa H. Genetic variation in the RNA transcripts of endogenous virus genes in uninfected chicken cells. J Virol. 1977 Oct;24(1):64–73. doi: 10.1128/jvi.24.1.64-73.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]



