Abstract
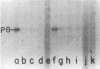
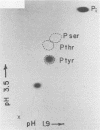
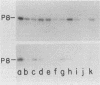
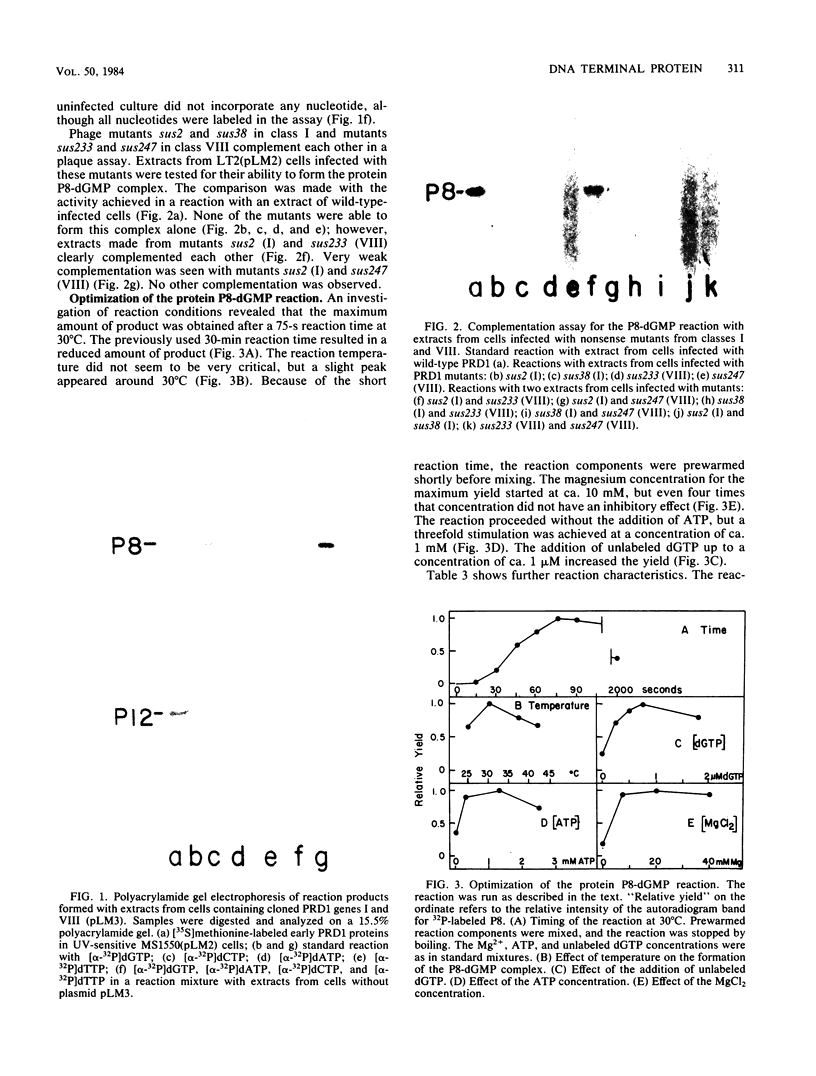
DNA of bacteriophage PRD1 has protein P8 at its termini. Extracts of infected cells are able to derivatize P8 in vitro with labeled dGTP. Two early proteins, P1 and P8, products of genes I and VIII, respectively, are the only phage proteins necessary for the formation of the protein P8-dGMP complex. This was shown by complementation of extracts from cells infected with mutants and by use of extracts from cells carrying cloned genes I and VIII. With Escherichia coli mutants that are temperature sensitive for DNA synthesis, it was possible to show that the formation of the protein P8-dGMP complex was dependent upon the host replication apparatus. The analysis of the purified protein P8-dGMP complex by hydrolysis and enzymatic digestion showed that there is a covalent phosphodiester bond between tyrosine and 5'-dGMP.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. L., Reilly B. E. Analysis of bacteriophage phi 29 gene function: protein synthesis in suppressor-sensitive mutant infection of Bacillus subtilis. J Virol. 1974 Jan;13(1):211–221. doi: 10.1128/jvi.13.1.211-221.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamford D. H., Rouhiainen L., Takkinen K., Söderlund H. Comparison of the lipid-containing bacteriophages PRD1, PR3, PR4, PR5 and L17. J Gen Virol. 1981 Dec;57(Pt 2):365–373. doi: 10.1099/0022-1317-57-2-365. [DOI] [PubMed] [Google Scholar]
- Bamford D., McGraw T., MacKenzie G., Mindich L. Identification of a protein bound to the termini of bacteriophage PRD1 DNA. J Virol. 1983 Aug;47(2):311–316. doi: 10.1128/jvi.47.2.311-316.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamford D., Mindich L. Structure of the lipid-containing bacteriophage PRD1: disruption of wild-type and nonsense mutant phage particles with guanidine hydrochloride. J Virol. 1982 Dec;44(3):1031–1038. doi: 10.1128/jvi.44.3.1031-1038.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coetzee W. F., Bekker P. J. Pilus-specific, lipid-containing bacteriophages PR4 and PR772: comparison of physical characteristics of genomes. J Gen Virol. 1979 Oct;45(1):195–200. doi: 10.1099/0022-1317-45-1-195. [DOI] [PubMed] [Google Scholar]
- Collett M. S., Purchio A. F., Erikson R. L. Avian sarcoma virus-transforming protein, pp60src shows protein kinase activity specific for tyrosine. Nature. 1980 May 15;285(5761):167–169. doi: 10.1038/285167a0. [DOI] [PubMed] [Google Scholar]
- Davis T. N., Cronan J. E., Jr Nonsense mutants of the lipid-containing bacteriophage PR4. Virology. 1983 Apr 30;126(2):600–613. doi: 10.1016/s0042-6822(83)80016-6. [DOI] [PubMed] [Google Scholar]
- Enomoto T., Lichy J. H., Ikeda J. E., Hurwitz J. Adenovirus DNA replication in vitro: purification of the terminal protein in a functional form. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6779–6783. doi: 10.1073/pnas.78.11.6779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumport R. I., Lehman I. R. Structure of the DNA ligase-adenylate intermediate: lysine (epsilon-amino)-linked adenosine monophosphoramidate. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2559–2563. doi: 10.1073/pnas.68.10.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermoso J. M., Salas M. Protein p3 is linked to the DNA of phage phi 29 through a phosphoester bond between serine and 5'-dAMP. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6425–6428. doi: 10.1073/pnas.77.11.6425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda J. E., Enomoto T., Hurwitz J. Adenoviral protein-primed initiation of DNA chains in vitro. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2442–2446. doi: 10.1073/pnas.79.8.2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichy J. H., Field J., Horwitz M. S., Hurwitz J. Separation of the adenovirus terminal protein precursor from its associated DNA polymerase: role of both proteins in the initiation of adenovirus DNA replication. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5225–5229. doi: 10.1073/pnas.79.17.5225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichy J. H., Horwitz M. S., Hurwitz J. Formation of a covalent complex between the 80,000-dalton adenovirus terminal protein and 5'-dCMP in vitro. Proc Natl Acad Sci U S A. 1981 May;78(5):2678–2682. doi: 10.1073/pnas.78.5.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K., Saito T., Hirokawa H. In vitro initiation of bacteriophage phi 29 and M2 DNA replication: genes required for formation of a complex between the terminal protein and 5'dAMP. Mol Gen Genet. 1983;191(1):26–30. doi: 10.1007/BF00330885. [DOI] [PubMed] [Google Scholar]
- McGraw T., Yang H. L., Mindich L. Establishment of a physical and genetic map for bacteriophage PRD1. Mol Gen Genet. 1983;190(2):237–244. doi: 10.1007/BF00330646. [DOI] [PubMed] [Google Scholar]
- Mindich L., Bamford D., Goldthwaite C., Laverty M., Mackenzie G. Isolation of nonsense mutants of lipid-containing bacteriophage PRD1. J Virol. 1982 Dec;44(3):1013–1020. doi: 10.1128/jvi.44.3.1013-1020.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mindich L., Bamford D., McGraw T., Mackenzie G. Assembly of bacteriophage PRD1: particle formation with wild-type and mutant viruses. J Virol. 1982 Dec;44(3):1021–1030. doi: 10.1128/jvi.44.3.1021-1030.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mindich L., Cohen J., Weisburd M. Isolation of nonsense suppressor mutants in Pseudomonas. J Bacteriol. 1976 Apr;126(1):177–182. doi: 10.1128/jb.126.1.177-182.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mindich L., McGraw T. Molecular cloning of bacteriophage PRD1 genomic fragments. Mol Gen Genet. 1983;190(2):233–236. doi: 10.1007/BF00330645. [DOI] [PubMed] [Google Scholar]
- Overbye K. M., Basu S. K., Margolin P. Loss of DNA topoisomerase I activity alters many cellular functions in Salmonella typhimurium. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 2):785–791. doi: 10.1101/sqb.1983.047.01.090. [DOI] [PubMed] [Google Scholar]
- Peñalva M. A., Salas M. Initiation of phage phi 29 DNA replication in vitro: formation of a covalent complex between the terminal protein, p3, and 5'-dAMP. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5522–5526. doi: 10.1073/pnas.79.18.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclafani R. A., Wechsler J. A. Growth of phages lambda and phiX174 under Plban protein control in the absence of host dnaB function. Virology. 1981 Aug;113(1):314–322. doi: 10.1016/0042-6822(81)90158-6. [DOI] [PubMed] [Google Scholar]
- Sinclair J. F., Cohen J., Mindich L. The isolation of suppressible nonsence mutants of bacteriophage phi6. Virology. 1976 Nov;75(1):198–208. [PubMed] [Google Scholar]
- Stillman B. W., Tamanoi F., Mathews M. B. Purification of an adenovirus-coded DNA polymerase that is required for initiation of DNA replication. Cell. 1982 Dec;31(3 Pt 2):613–623. doi: 10.1016/0092-8674(82)90317-8. [DOI] [PubMed] [Google Scholar]
- Stillman B. W. The replication of adenovirus DNA with purified proteins. Cell. 1983 Nov;35(1):7–9. doi: 10.1016/0092-8674(83)90201-5. [DOI] [PubMed] [Google Scholar]
- Watabe K., Shin M., Ito J. Protein-primed initiation of phage phi 29 DNA replication. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4248–4252. doi: 10.1073/pnas.80.14.4248. [DOI] [PMC free article] [PubMed] [Google Scholar]