Abstract
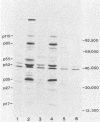
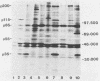
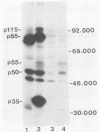
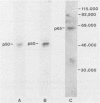
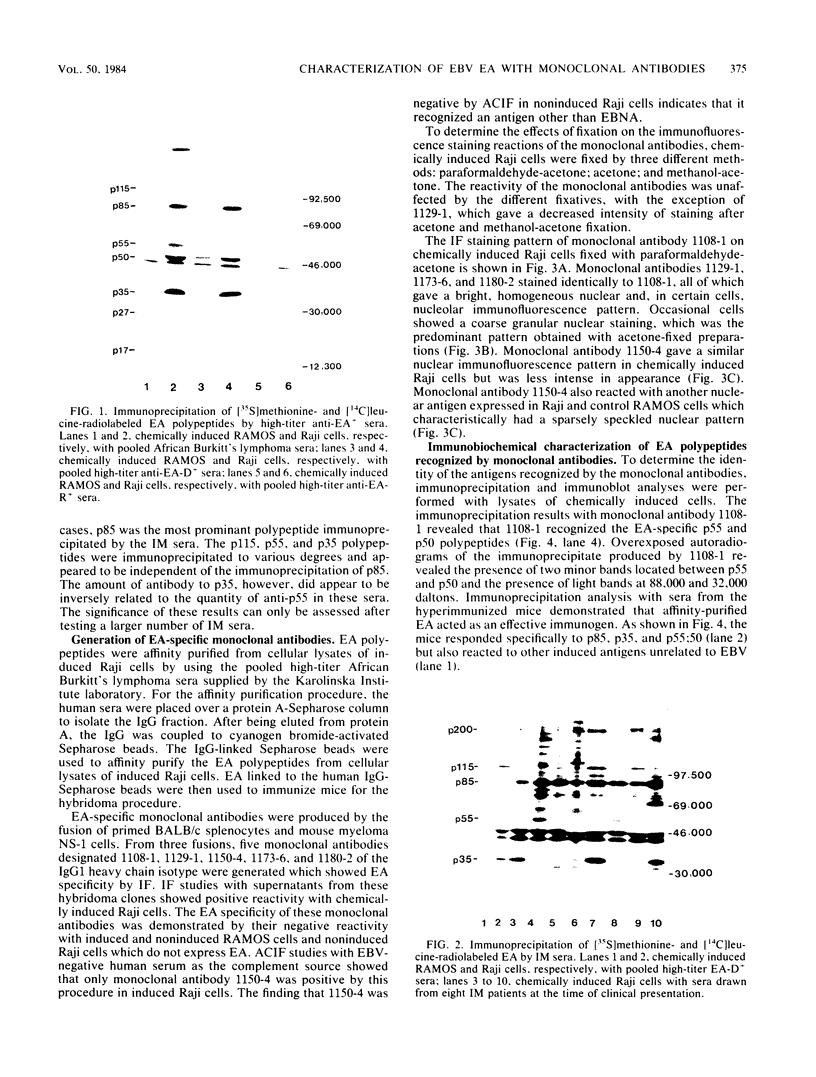
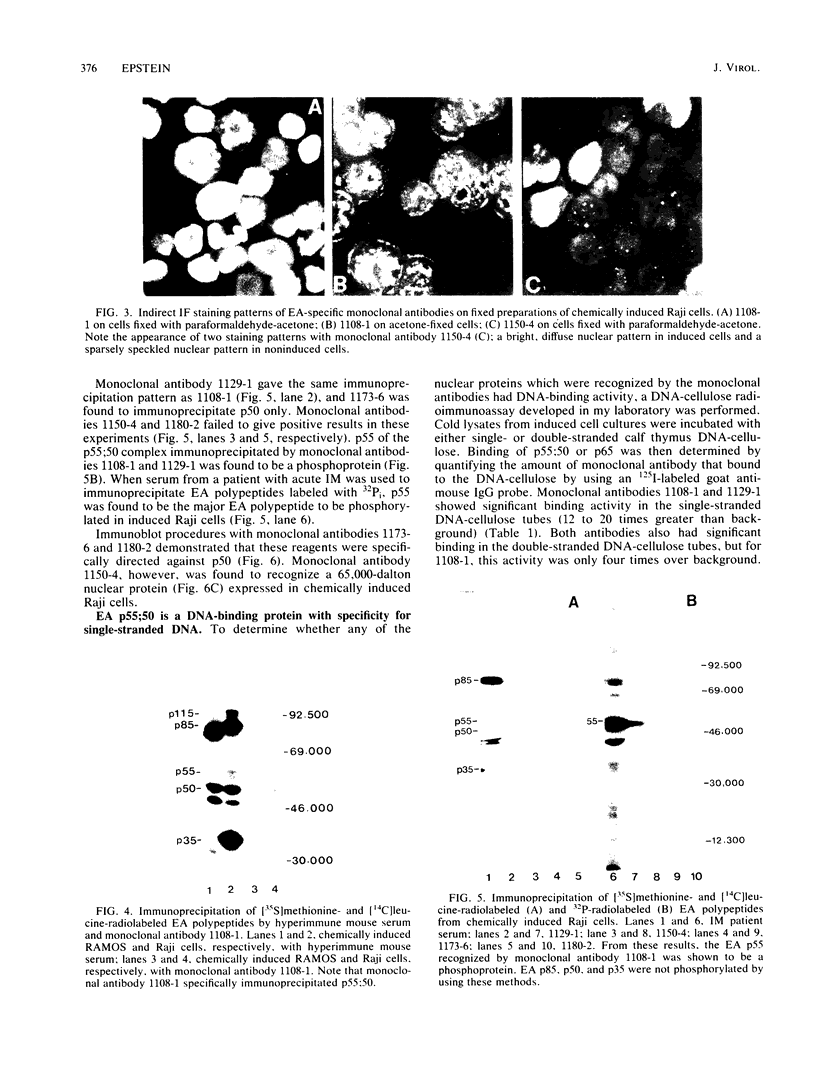
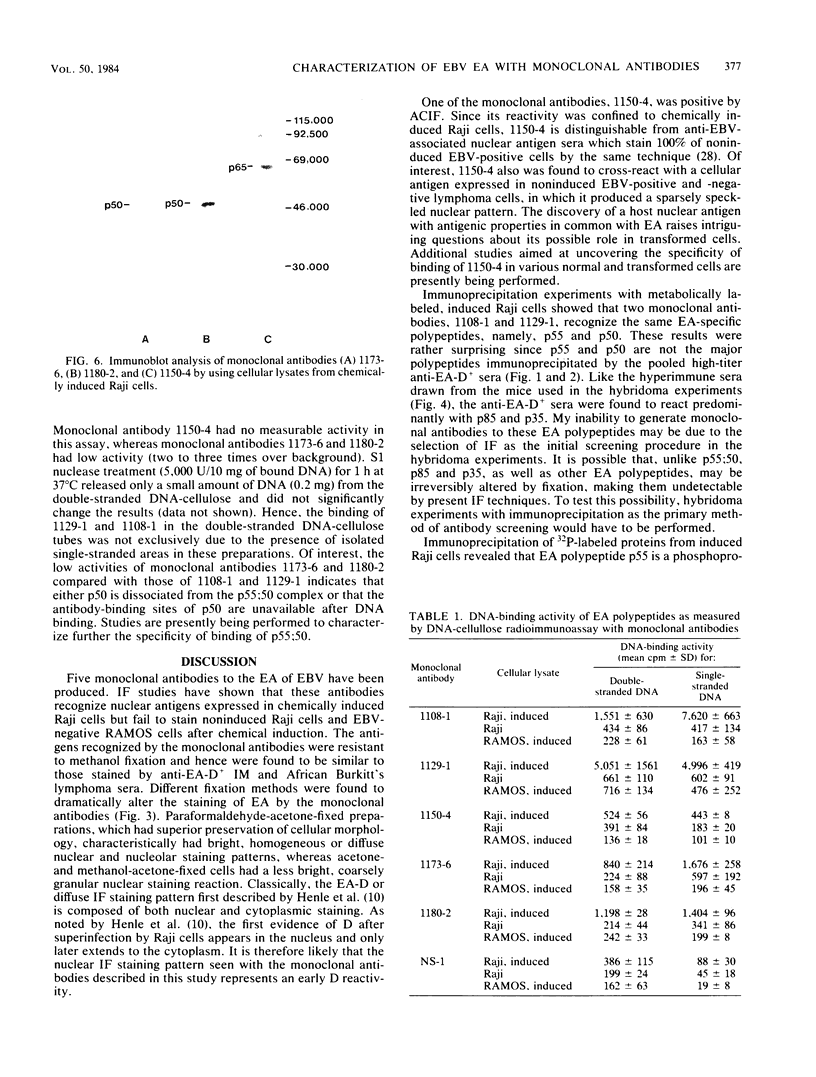
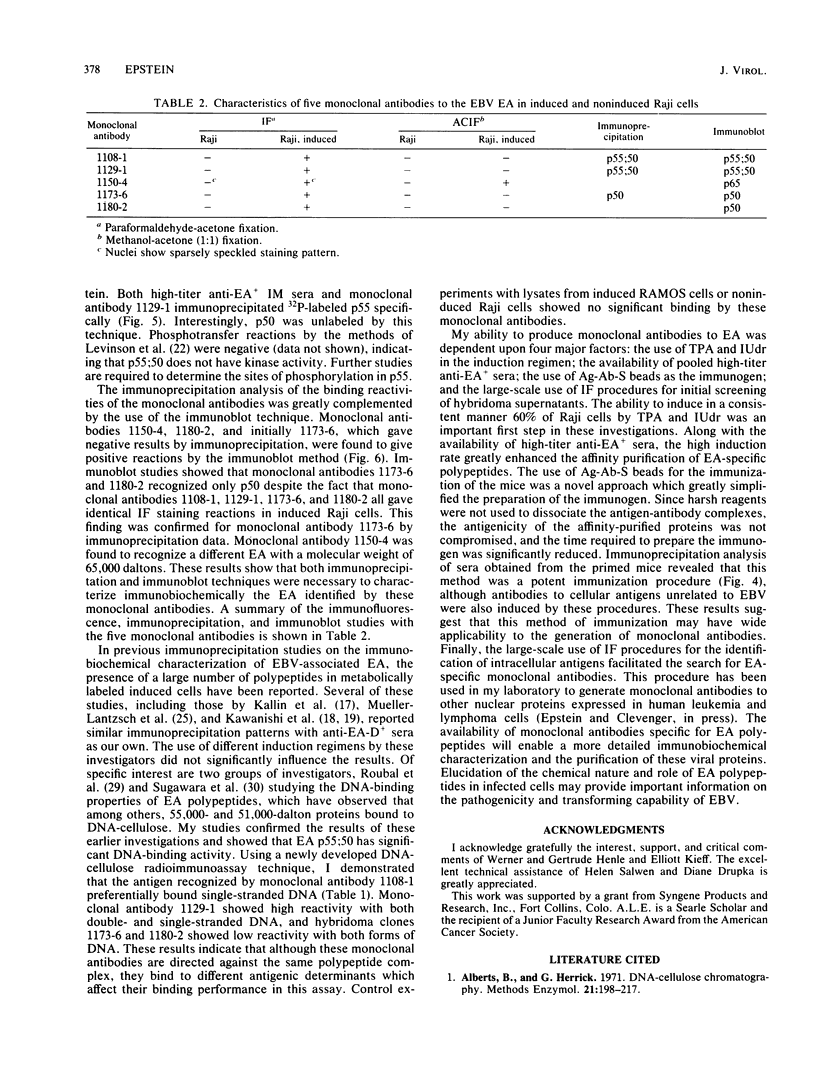
Five monoclonal antibodies which are reactive to early antigens of Epstein-Barr virus have been produced by using somatic cell hybridization techniques. The specificity of the monoclonal antibodies to early antigens was demonstrated by indirect immunofluorescence, which showed that the antigens were localized to the nucleus of early antigen-induced Raji cells. Additional indirect immunofluorescence studies showed that like patient antisera to diffuse-staining early antigen, the monoclonal antibodies gave positive staining reactions after methanol fixation. One of the antibodies, 1150-4, was positive by the anti-complement immunofluorescence technique but differed with Epstein-Barr virus-associated nuclear antigen-positive patient sera in that it only stained induced cells. Different fixation methods were found to alter dramatically the appearance of the nuclear staining reactions produced by the monoclonal antibodies. Immunoprecipitation and immunoblot experiments revealed that monoclonal antibodies 1108-1 and 1129-1 recognized two polypeptides of 55,000 and 50,000 daltons (p55;50), 1173-6 and 1180-2 recognized just p50, and 1150-4 identified a 65,000-dalton nuclear protein. Immunobiochemical characterization of these viral antigens showed that p55 is a phosphoprotein, and p55;50 has strong DNA-binding activity preferentially to single-stranded DNA. Elucidation of the role of these nuclear proteins in Epstein-Barr virus infection and the events associated with Epstein-Barr virus-directed lymphocyte transformation may provide significant information on the pathogenicity of this important human virus.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayliss G. J., Nonoyama M. Mechanisms of infection with Epstein-Barr virus. III. The synthesis of proteins in superinfected Raji cells. Virology. 1978 Jun 1;87(1):204–207. doi: 10.1016/0042-6822(78)90173-3. [DOI] [PubMed] [Google Scholar]
- Bodemer W. W., Summers W. C., Niederman J. C. Detection of virus-specific antigens in EB-(P3HR1) virus-superinfected Raji cells by immunoprecipitation. Virology. 1980 Jun;103(2):340–349. doi: 10.1016/0042-6822(80)90192-0. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Edson C. M., Thorley-Lawson D. A. Epstein-Barr virus membrane antigens: characterization, distribution, and strain differences. J Virol. 1981 Jul;39(1):172–184. doi: 10.1128/jvi.39.1.172-184.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feighny R. J., Henry B. E., 2nd, Pagano J. S. Epstein-Barr virus polypeptides: identification of early proteins and their synthesis and glycosylation. J Virol. 1981 Aug;39(2):651–655. doi: 10.1128/jvi.39.2.651-655.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber P. Activation of Epstein-Barr virus by 5-bromodeoxyuridine in "virus-free" human cells (complement-fixing antigen-immunofluorescence-leukocytes). Proc Natl Acad Sci U S A. 1972 Jan;69(1):83–85. doi: 10.1073/pnas.69.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gergely L., Klein G., Ernberg I. The action of DNA antagonists on Epstein-Barr virus (EBV)-associated early antigen (EA) in Burkitt lymphoma lines. Int J Cancer. 1971 Mar 15;7(2):293–302. doi: 10.1002/ijc.2910070214. [DOI] [PubMed] [Google Scholar]
- Henle G., Henle W. Immunofluorescence in cells derived from Burkitt's lymphoma. J Bacteriol. 1966 Mar;91(3):1248–1256. doi: 10.1128/jb.91.3.1248-1256.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henle G., Henle W., Klein G. Demonstration of two distinct components in the early antigen complex of Epstein-Barr virus-infected cells. Int J Cancer. 1971 Sep 15;8(2):272–282. doi: 10.1002/ijc.2910080212. [DOI] [PubMed] [Google Scholar]
- Henle G., Henle W., Klein G., Gunven P., Clifford P., Morrow R. H., Ziegler J. L. Antibodies to early Epstein-Barr virus-induced antigens in Burkitt's lymphoma. J Natl Cancer Inst. 1971 Apr;46(4):861–871. [PubMed] [Google Scholar]
- Henle W., Henle G. E., Horwitz C. A. Epstein-Barr virus specific diagnostic tests in infectious mononucleosis. Hum Pathol. 1974 Sep;5(5):551–565. doi: 10.1016/s0046-8177(74)80006-7. [DOI] [PubMed] [Google Scholar]
- Henle W., Henle G. Effect of arginine-deficient media on the herpes-type virus associated with cultured Burkitt tumor cells. J Virol. 1968 Mar;2(3):182–191. doi: 10.1128/jvi.2.3.182-191.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henle W., Henle G., Zajac B. A., Pearson G., Waubke R., Scriba M. Differential reactivity of human serums with early antigens induced by Epstein-Barr virus. Science. 1970 Jul 10;169(3941):188–190. doi: 10.1126/science.169.3941.188. [DOI] [PubMed] [Google Scholar]
- Ito Y., Kishishita M., Yanase S. Induction of Epstein-Barr virus antigens in human lymphoblastoid P3HR-1 cells with culture fluid of Fusobacterium nucleatum. Cancer Res. 1980 Nov;40(11):4329–4330. [PubMed] [Google Scholar]
- Kallin B., Luka J., Klein G. Immunochemical characterization of Epstein-Barr virus-associated early and late antigens in n-butyrate-treated P3HR-1 cells. J Virol. 1979 Dec;32(3):710–716. doi: 10.1128/jvi.32.3.710-716.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawanishi M., Sugawara K., Ito Y. Epstein-Barr virus-induced early polypeptides in Raji and NC37 cells activated by diterpene ester TPA in combination with N-butyrate. Virology. 1981 Dec;115(2):406–409. doi: 10.1016/0042-6822(81)90123-9. [DOI] [PubMed] [Google Scholar]
- Kawanishi M., Sugawara K., Ito Y. Epstein-Barr virus-induced polypeptides: a comparative study with superinfected Raji, IUdR-Treated, and N-butyrate-treated P3HR-1 cells. Virology. 1981 Feb;109(1):72–81. doi: 10.1016/0042-6822(81)90472-4. [DOI] [PubMed] [Google Scholar]
- Klein G., Giovanella B. C., Lindahl T., Fialkow P. J., Singh S., Stehlin J. S. Direct evidence for the presence of Epstein-Barr virus DNA and nuclear antigen in malignant epithelial cells from patients with poorly differentiated carcinoma of the nasopharynx. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4737–4741. doi: 10.1073/pnas.71.12.4737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levinson A. D., Oppermann H., Levintow L., Varmus H. E., Bishop J. M. Evidence that the transforming gene of avian sarcoma virus encodes a protein kinase associated with a phosphoprotein. Cell. 1978 Oct;15(2):561–572. doi: 10.1016/0092-8674(78)90024-7. [DOI] [PubMed] [Google Scholar]
- Luka J., Kallin B., Klein G. Induction of the Epstein-Barr virus (EBV) cycle in latently infected cells by n-butyrate. Virology. 1979 Apr 15;94(1):228–231. doi: 10.1016/0042-6822(79)90455-0. [DOI] [PubMed] [Google Scholar]
- Moss L. G., Moore J. P., Chan L. A simple, efficient method for coupling DNA to cellulose. Development of the method and application to mRNA purification. J Biol Chem. 1981 Dec 25;256(24):12655–12658. [PubMed] [Google Scholar]
- Mueller-Lantzsch N., Yamamoto N., zur Hausen H. Analysis of early and late Epstein-Barr virus associated polypeptides by immunoprecipitation. Virology. 1979 Sep;97(2):378–387. doi: 10.1016/0042-6822(79)90348-9. [DOI] [PubMed] [Google Scholar]
- Nyormoi O., Thorley-Lawson D. A., Elkington J., Strominger J. L. Differential effect of phosphonoacetic acid on the expression of Epstein-Barr viral antigens and virus production. Proc Natl Acad Sci U S A. 1976 May;73(5):1745–1748. doi: 10.1073/pnas.73.5.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reedman B. M., Klein G. Cellular localization of an Epstein-Barr virus (EBV)-associated complement-fixing antigen in producer and non-producer lymphoblastoid cell lines. Int J Cancer. 1973 May;11(3):499–520. doi: 10.1002/ijc.2910110302. [DOI] [PubMed] [Google Scholar]
- Roubal J., Kallin B., Luka J., Klein G. Early DNA-binding polypeptides of Epstein-Barr virus. Virology. 1981 Aug;113(1):285–292. doi: 10.1016/0042-6822(81)90155-0. [DOI] [PubMed] [Google Scholar]
- Sugawara K., Kawanishi M., Ito Y. Epstein-barr virus-related DNA-binding proteins induced by n-butyrate in P3HR-1 cells. Virology. 1982 Jan 15;116(1):354–358. doi: 10.1016/0042-6822(82)90427-5. [DOI] [PubMed] [Google Scholar]
- Summers W. C., Klein G. Inhibition of Epstein-Barr virus DNA synthesis and late gene expression by phosphonoacetic acid. J Virol. 1976 Apr;18(1):151–155. doi: 10.1128/jvi.18.1.151-155.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovey M. G., Lenoir G., Begon-Lours J. Activation of latent Epstein-Barr virus by antibody to human IgM. Nature. 1978 Nov 16;276(5685):270–272. doi: 10.1038/276270a0. [DOI] [PubMed] [Google Scholar]
- Yamamoto N., zur Hausen H. Induction of Epstein-Barr virus early antigens by intercalating chemicals in B95-8 cells. Virology. 1981 Dec;115(2):390–394. doi: 10.1016/0042-6822(81)90120-3. [DOI] [PubMed] [Google Scholar]
- Zur Hausen H., Schulte-Holthausen H. Presence of EB virus nucleic acid homology in a "virus-free" line of Burkitt tumour cells. Nature. 1970 Jul 18;227(5255):245–248. doi: 10.1038/227245a0. [DOI] [PubMed] [Google Scholar]
- zur Hausen H., Bornkamm G. W., Schmidt R., Hecker E. Tumor initiators and promoters in the induction of Epstein-Barr virus. Proc Natl Acad Sci U S A. 1979 Feb;76(2):782–785. doi: 10.1073/pnas.76.2.782. [DOI] [PMC free article] [PubMed] [Google Scholar]