Abstract
Diversity of the primary groups of contemporary Indo-West Pacific coral reef organisms, including mantis shrimps (stomatopod crustaceans), peaks in the Indo-Australian Archipelago (IAA), reaches a lower peak in East Africa and Madagascar [Indian Ocean continental (IOC)], and declines in the central Indian Ocean (IO) and Central Pacific (CP). Percent endemism in stomatopods (highest in the IAA, high in the IOC, lower in regions adjacent to centers, and moderate in the CP) correlates positively with species diversity (this varies with scale) and inversely with species body size. Because it constrains reproductive traits and dispersal, body size is a reliable indicator of speciation and extinction potential in reef stomatopods and probably most marine organisms. Assemblages are dominated by small-sized species in the IAA and IOC. Both speciation and extinction likely are high, resulting in especially high endemism (small ranges reflect both originating and disappearing species) in these regions. Rates of speciation exceed extinction, yielding centers of diversity (especially in the IAA). Dispersal slows speciation and extinction in areas adjacent to these centers. Body size declines toward the CP, especially in atoll environments. Here the wheels of speciation and extinction again spin rapidly but in the opposite direction (extinction > speciation), yielding low diversity and moderate endemism. We conclude that life histories, dispersal, and speciation/extinction dynamics are primary agents that mold patterns of diversity and endemism. Historical factors, currents, productivity, and species diversity itself (through ecological interactions) also influence these patterns, in some cases by altering body size.
Keywords: hot spots, stomatopod, life history, geographic range, body size
Being repositories of ancient phyla as well as more recent specialized taxa, coral reefs are among the most spectacular, productive, diverse (per unit area), and threatened ecosystems on earth. Organisms they house provide a critical source of protein for people in many tropical countries, and reefs themselves protect human populations from storm and wave damage. Coral reefs provide aesthetic beauty (and the bioeroded sand on beaches) for tourism—an increasingly important economic resource for developing tropical countries. However, tourism and other uses of reefs must be carefully managed to be of sustainable economic benefit. A fundamental value of coral reefs is that they provide an aesthetic, intellectual, educational, and cultural heritage for present and future generations.
Threats to global coral reefs, however, are severe and well documented (1–9). Overexploitation has been identified as an especially serious problem, but other threats include coastal development and sedimentation, pollution, global warming, disease, and ocean acidification. The Global Coral Reef Monitoring Network reports that 20% of global coral reefs already have been degraded beyond recovery, an additional 24% are under imminent threat of collapse, and a further 26% are at longer-term risk (10). In the Indo-West Pacific (IWP), 88% of Southeast Asian reefs and 61% and 54% of Middle Eastern and Indian Ocean (IO) reefs are at medium to high risk (1, 2). Human population density near reefs is particularly high in Southeast Asia and the IO. Pacific reefs are in better condition (59% at low risk), with more protected area, than those in other regions.
Despite the biological, cultural, and economic value of coral reefs and the widely publicized alarm at their global decline, it remains astonishing how little we know about the patterns of diversity (“diversity” will be used equivalently to species richness) on coral reefs that would help us manage and conserve them. This article will (i) review and provide new information on the geography of coral reef diversity in the IWP using information from the most ecologically important and well known groups of reef organisms, (ii) provide a brief overview of the major factors that generate these patterns, and (iii) briefly review “hotspots” and provide new information on endemism in reef stomatopods. (iv) We will then examine information on body size, life history characteristics, geographic ranges, and speciation/extinction dynamics of reef stomatopods and other organisms to suggest mechanisms that, in combination with environmental factors, can explain the observed patterns of IWP coral reef diversity and endemism.
We will focus on reef-dwelling mantis shrimps as a model taxonomic group for such analyses because these crustaceans are important members of the benthic community. All stomatopods are predators with a pair of enlarged, equally sized raptorial claws that are used to smash and spear prey, competitors, and predators (Fig. 1). Protective holes in the substrate are a limiting resource because of strong fish predation; stomatopods exhibit colorful communicatory displays and intense territorial fighting to maintain exclusive ownership of these holes (11, 12).
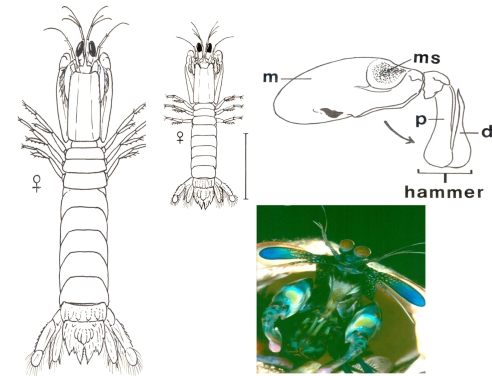
Fig. 1.
Dorsal view of typically sized reef stomatopods (large Gonodactylaceus falcatus, small Gonodactylellus incipiens; scale bar for both is 10.0 mm), lateral view of raptorial claw, and photograph of individual delivering a threatening display at the entrance of its burrow (Gonodactylaceus ternatensis, courtesy of Roy Caldwell). Drawing of the raptorial claw shows the merus (m), meral spot (ms, varies in color from white through yellow, orange, red, pink, purple, and blue among species), propodus (p), and dactyl (d); p and d normally are folded against m but are opened (arrow) either together as a hammer or with d projected as a spear. The photograph shows species-specific coloration of the flared antennal scales (blue) and setae (red) and spread raptorial claws of the display (yellow ms surrounded by white ring).
Patterns of Coral Reef Diversity
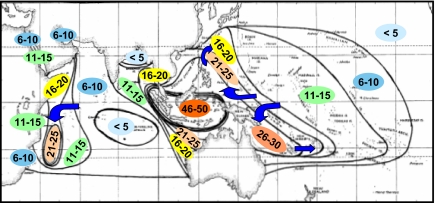
Species diversity for several marine taxa (fishes, corals, lobsters, and snails) reaches a global maximum in the “East Indies triangle” (Malaysia, Indonesia, New Guinea, and the Philippines) of the Indo-Australian Archipelago (IAA), declines in the IO (with heightened diversity in some parts of the western IO for many taxa), declines eastward across the Central Pacific (CP), and peaks again in the Caribbean (13–26). The first diversity contour map for reef stomatopods (Fig. 2) shows a similar pattern, with a high, sharp peak in the central IAA and an area of elevated diversity in the northwestern IO that increases southward to a secondary peak around Madagascar. Similar to most other taxa where contours of diversity are known, tails of diversity extend from the IAA toward the southeast and northeast.
Fig. 2.
Contours of species richness for reef stomatopods in the IWP. Numbers represent species present in each contour. Arrows indicate major currents. All species of Alainosquillidae, Gonodactylidae, Odtontodactylidae, Protosquillidae, and Takuidae are included; Pseudosquillidae occur on reefs but are excluded from analysis because their reproductive, larval, and life history patterns differ from those of other reef-dwelling families (27–29). Data are from our own collections, National Museum of Natural History collections, and published literature [updated to currently accepted taxonomy (30, 31)].
Explanations of IWP Diversity Gradients
After considering all explanations for patterns of IWP reef biodiversity, we identify here only those that are most applicable to the present study.
Faunal Carryover Hypothesis.
Species from a Mesozoic/early Cenozoic center of diversity in Europe migrated east to the IAA and probably south along the continental margin of the Indian Ocean (IOC) as the Tethys Seaway was closed by the collision of Africa with Eurasia between the Paleocene and Miocene (17–19). High stomatopod diversity in the IAA and in the western/southwestern IO (Fig. 2) is consistent with this hypothesis.
Center of Accumulation Hypothesis.
Species originate in small peripheral populations, larvae from peripheral regions are carried by currents into central areas favorable for reef growth (arrows in Fig. 2), and species accumulate in these current-fed regions over time (15, 32–34). Peaks of stomatopod diversity in the IAA and western IO are consistent with this hypothesis, but Barber and Bellwood (35) and the present study find speciation and endemism in both peripheral regions and diversity centers.
Energy/Productivity Hypothesis.
Higher productivity—the rate at which energy flows through an ecosystem—allows an ecosystem to support more species (although diversity often declines at very high levels of productivity) (36). Similarly, increased temperature accelerates speciation (37, 38), but Bellwood et al. (26) find no relationship between sea surface temperature and diversity of reef corals and fishes. Phytoplankton abundance has not been compared previously with contours of reef diversity. The general pattern of stomatopod diversity correlates fairly well with phytoplankton productivity (Figs. 2 and 3). We later infer that phytoplankton productivity affects body size and extinction/speciation dynamics of stomatopods on high (volcanic peaks with extensive terrestrial area and soil) vs. low (carbonate atolls with little elevation or nutrient runoff) mid-Pacific islands. The fact that terrestrial runoff elevates productivity around high vs. low Pacific islands, enhancing the survival of phytoplankton-feeding starfish larvae and fostering Crown of Thorns starfish population explosions (39–41), suggests that productivity can have important effects on larval recruitment, local reef populations, and hence reef biodiversity.
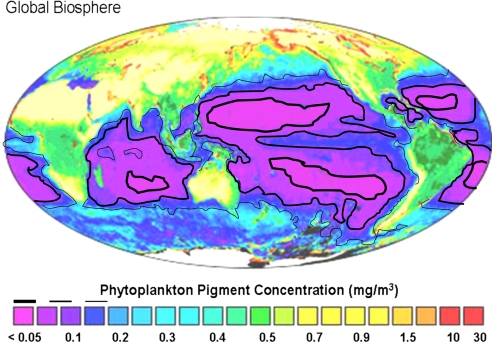
Fig. 3.
Distribution of global phytoplankton pigment concentration (adapted with permission from the National Aeronautics and Space Administration). Among reef areas, phytoplankton pigments are particularly abundant (0.3–0.4 mg/m3) off the Arabian Peninsula and west coast of India/Sri Lanka, around the Malay Peninsula/Indonesia, and around New Guinea/northern Australia. Pigment concentrations decline to 0.15 mg/m3 in a relatively narrow band oceanward from the above areas; immediately offshore from the continental margin of western Australia, eastern Africa, Madagascar; and in an equatorial band extending westward from the central East Pacific. Still further offshore, phytoplankton pigments decline (0.05 mg/m3) until they reach the very low levels characteristic of the centers of the northern and southern gyres of the Indian and Pacific Oceans (<0.05 mg/m3, smallest oceanic circles). We drew black lines for each of these contours by eye from map coloration.
Center of Origin Hypothesis.
“Successful” lineages originate in the East Indies; species subsequently migrate into peripheral regions, where they remain as relicts; gaps in species distributions suggest high extinction as well as origination in the East Indies (15–19, 42). Although much evidence supports this hypothesis, species of reef fish are not consistently young in the IAA and old in peripheral regions (35), and the present study suggests that, in reef stomatopods, both speciation and extinction are high in peripheral areas as well as in the IAA.
Species Diversity Hypothesis.
High species diversity itself may promote diversification (ref. 43, but see ref. 44), probably through species interactions. Speciation rates in three groups of fossil plankton over 2–20 my correlate with species diversity independent of sampling intensity and area (38). In the present study, species interactions in diverse assemblages may cause shifts in body size and consequent changes in life history patterns and speciation/extinction patterns.
Life History Speciation/Extinction Hypothesis.
We will suggest that the biotic and environmental processes that govern body size and life history traits drive rates of speciation/extinction and thus patterns of diversity in IWP stomatopods and other reef organisms.
Endemism and Hotspots
Endemism has been of particular interest as an indicator of extinction. The concept of biodiversity hotspots—concentrations of endemic species that are at exceptional risk—was motivated by the need to establish conservation priorities (45–48). However, some authors have argued that high overall diversity or phylogenetically unique taxa or habitats deserve priority attention, and others have shown that centers of endemism do not always coincide among taxa or with degree of threat (49–52). For some of the same reasons, hotspots on global coral reefs have been controversial (22, 23, 53, 54). Here we examine patterns of endemism in the context of both speciation and extinction, because limited ranges occur during both processes. Conservation of areas rich in endemics is important not only because they are at particular risk of species loss but also because they represent potential sources of diversification.
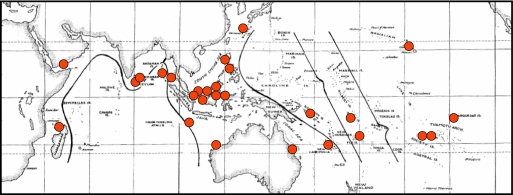
Endemism in reef stomatopods varies with scale. When stomatopods are known from only a single locality, these “local endemics” are widely scattered (Fig. 4), with no significant relationship between number of endemics and number of species found at each local site (x2 = 12.72, df = 20, P > 0.05). Average endemism for the 21 local sites is 10.5 ± 2.6% (SE).
Fig. 4.
“Locally endemic” species of reef stomatopods.
“Restricted regional endemics”—those known from local sites, archipelagos, and parts of regions—are widespread but reach their highest incidences (>15%) in the Malay Peninsula/Indonesia/Philippines, Red Sea, Mascarene Islands, Madagascar/Comores Islands, Society Islands, and Hawaiian Islands [see supporting information (SI) Fig. S1]. However, the distribution of restricted regional endemics does not differ from those expected when the diversities of their subregional stomatopod faunas are considered (x2 = 25.13, df = 23, P > 0.05). Endemism across the 24 subregions averages 14.5 ± 2.1%.
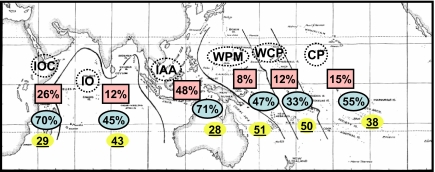
To examine patterns of endemism at a larger scale, the IWP was divided into major “regions” of continental and oceanic habitat types. At this scale, percent endemism (squares) is significantly concentrated in the IAA and drops in the adjacent oceanic regions but then rises toward the west in the IOC and, to a lesser extent, toward the east in the West Central Pacific and especially the CP (x2 = 26.23 using raw numbers of endemics/nonendemics, df = 5, P < 0.001; Fig. 5). Average endemism among the six regions is 20.2 ± 6.1%.
Fig. 5.
Endemism and body size of reef stomatopods in six different regions (dashed circles) consisting of the IOC, IO, IAA (the western margin of the IAA abuts the IOC off Burma), Western Pacific margin (WPM), West Central Pacific (WCP), and CP. Numbers in each region represent (i) percentage of species in each region that are endemic (square, top number in each region; percentage is used to avoid confounding diversity with endemism and because the regions are not equal in area), (ii) percentage of species in each region <40 mm in body length (oval, middle number in each region; 40 mm is the median body size across all regions), and (iii) median body size (millimeters total length) among species in each region (underline bar, bottom number in each region).
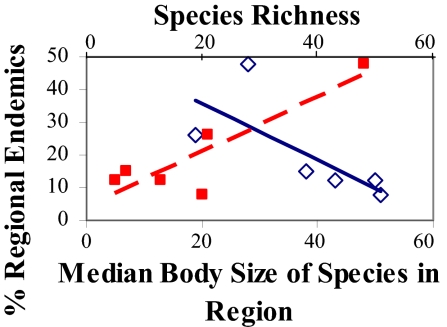
The percent regional endemism increases significantly with regional species richness (Fig. 6; r2 = 0.74, P = 0.03, F = 11.41, y = 0.08x + 1.61, square-root-transformed data).
Fig. 6.
The percentage of regional endemics vs. median body size of species in each region (open diamonds) and species richness in each region (filled squares) for reef stomatopods. Residual analyses show no difference in and statistical results are similar for transformed and untransformed data; untransformed data are shown here.
The percent regional endemism is inversely related to body size among regions, decreasing with increased median body size (Fig. 6; r2 = 0.65, P = 0.05, F = 7.53, y = 0.10x + 8.16, square-root-transformed data) and increasing with percentage of species in each region that are <40 mm in body size (r2 = 0.68, P = 0.04, F = 8.39, y = 0.08x − 0.15, square-root-transformed data).
Species are concentrated in small body size classes in the IAA and IOC, although the range of body sizes is large in these regions. Typical body sizes are larger in the oceanic regions adjacent to the IAA and IOC but decline (with an absence of large sized species) toward the CP (Fig. 5; number of species >40 mm and <40 mm for all regions, x2 = 11.07, df = 5, P = 0.02).
Hypothesizing that productivity influences body sizes and life histories of reef stomatopods, we further categorized the species in the six regions according to whether they inhabited productive or unproductive environments. Because of terrestrial runoff, continental regions and high islands are expected to have higher nearshore productivity than low oceanic islands. Analysis of maximal species' body sizes on continental and high vs. low island environments shows, as above, that endemism is consistently associated with small body size (F = 39.27, df = 157, P < 0.001, type 3 ANOVA tests of fixed effects for regional endemism, terrain height, and region, log-transformed data; analysis using species median body size yields the same result). Terrain height is not consistently related to body size across all six regions, probably because of the large species scattered throughout the IOC, IO, and IAA (type 3 ANOVA of fixed effects as above, P > 0.05; for number of species >40 mm and <40 mm on continental/high vs. low islands across the region, x2 = 15.3, df = 9, P = 0.08). However, a previous study of reef stomatopods showed that body size of individual populations within each of four species complexes of reef stomatopods declines significantly from the IAA toward the CP and that populations on high islands reach significantly larger body sizes than those inhabiting atolls in these regions (55). Guided by the previous study, we analyzed maximal and median body sizes of species assemblages from high vs. low islands from the West Central Pacific and CP. High islands support species of significantly larger body size than low islands in these regions (F = 4.79, df = 40, P = 0.03; type 3 ANOVA of fixed effects for region, island height, and regional endemism, log-transformed data; analysis of median body size of species yields a similar result).
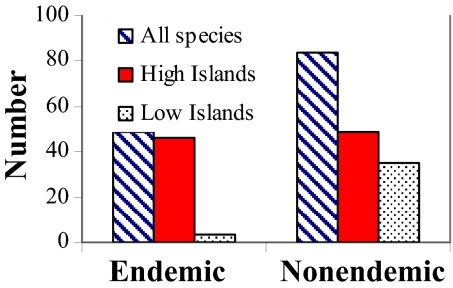
Number of endemics and nonendemics on high vs. low islands did not vary significantly across regions (P > 0.05), so all regions were combined. Significantly fewer endemics occur on low than high islands (x2 = 19.16, df = 1, P < 0.001; Fig. 7).
Fig. 7.
Number of regional endemic vs. nonendemic species of reef stomatopods and those occurring on high vs. low islands.
Life History Patterns of Reef Stomatopods
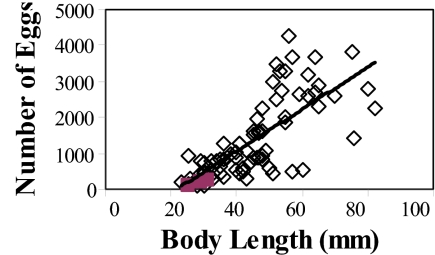
We propose that the ecological and environmental factors that govern body size and life history traits drive patterns of diversity and endemism in reef organisms. Large body size in reef stomatopods is significantly correlated with massive reproductive output (more and larger eggs; Fig. 8; see also Fig. S2), greater planktonic larval dispersal, larger geographic ranges, and greater saturation of available reef habitat within ranges (Fig. 9), whereas small body size correlates with restricted reproductive capacity, restricted larval dispersal, and relative rarity (low abundance, few sites, small geographic ranges) (11, 27–29, 56). This correlation occurs because small body size constrains reproductive traits in marine organisms. Small-bodied species cannot produce enough small plankton-feeding larvae to leave one surviving offspring given the high mortality rate suffered by these long-lived larvae. Small species must endow fewer, larger larvae with yolk supply, often brooding them before a relatively brief planktonic period, to increase survivorship. The body volume of larger species allows them to produce sufficient numbers of small larvae that feed in the plankton for long periods that some offspring survive despite heavy mortality (57, 58). Size frequency distributions for both body size (Fig. 10) and geographic range (Fig. 11) are strongly shifted toward diminutive sizes (particularly in endemic species) and restricted distributions in reef stomatopods. The latter indicates that most reef stomatopods risk extinction if faced with rapid global environmental changes.
Fig. 8.
Egg number per individual increases significantly with body size among species of gonodactylid (open diamonds) and protosquillid (filled squares) reef stomatopods (r2 = 0.55, F = 92.74, P < 0.001, y = 58.71x − 1291.69).
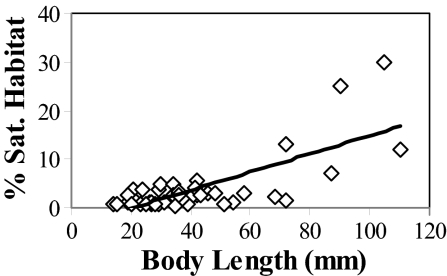
Fig. 9.
The percentage saturation of available reef habitat within each species' geographic range increases significantly with median body size among species of gonodactylid and protosquillid stomatopods (r2 = 0.54, F = 47.68, P < 0.001, y = 0.19x − 3.83). The percentage saturation of each species' range is the proportion of all 5 × 5° latitudinal and longitudinal quadrants containing habitable reef that is occupied by that species.
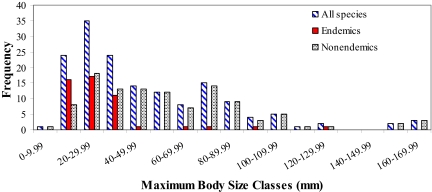
Fig. 10.
Size frequency distributions of maximal body sizes for total species, regional endemics, and nonendemic species of reef stomatopods. Endemics are significantly smaller than nonendemics (t = 6.40, df = 146, P < 0.001; two-sample t test assuming unequal variance). Analysis of median body size for each species gives a similar graphical and statistical result.
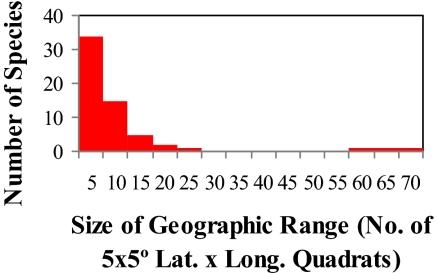
Fig. 11.
Size frequency distribution of geographic ranges in reef stomatopods (Lat, latitude; Long, longitude). Other measures of geographic range size [latitudinal distance, longitudinal distance, square root of (latitude × longitude)2] yield a similar plot.
Life history patterns of stomatopods are consistent with those found in other living and fossil groups for reproductive and life history traits, patterns of abundance, and frequency distributions of body size and geographic range. Body size is positively correlated with fecundity and colonizing ability in most marine invertebrates (27, 28, 56, 58–66). Stomatopods share the following characteristics with taxa in both marine and terrestrial environments. (i) Body size frequency distributions are usually shifted toward small size classes (36, 65, 67–71). (ii) Species abundances are biased toward few common and many rare species (72). (iii) Local abundance increases with range size (73). (iv) Geographic range size increases with body size (74). (v) Frequency distributions for geographic ranges are shifted toward small ranges (refs. 73 and 75–77, but see ref. 22). Commonalities in patterns of body size, life history, and distribution between reef stomatopods and other taxa suggest that the relationship between life history mechanisms and patterns of diversity and endemism we find in benthic reef organisms also may operate in other systems.
Speciation and Extinction
Factors that influence the relative rates of speciation vs. extinction control the geography of species diversity and endemism. This section will review briefly some of the factors thought to determine rates of extinction and speciation in marine and other organisms, and Discussion will apply these generalities to the patterns of diversity and endemism observed in IWP reef stomatopods.
Species or genera that are widespread, abundant, and dispersive resist extinction in both marine and terrestrial environments and both fossil and contemporary lineages (60, 66, 72, 73, 78–80). In addition to geographic range, which is sufficient on its own to explain species survival (81), the presence of long-lived larvae and species richness of the clade confer resistance to background extinction in fossil marine bivalves (78, 80). Broad distribution of the clade confers protection against mass extinction (78, 80, 82, 83). In addition, latitudinal distribution affects extinction, with the tropics—especially reef faunas—being subject to repeated upheaval, particularly during mass extinctions (80, 84, 85).
In stomatopods, evidence of extinction is derived from conspicuous gaps in regional distributions. For example, when a lineage occurs in the IWP, East Atlantic, and East Pacific but is absent from the West Atlantic, the most parsimonious explanation, given what is known about Cenozoic extinctions in the Americas (86–89), is that it became extinct in the West Atlantic. Such “apparent extinctions” are significantly elevated in lineages with restricted dispersal and small ranges as well as those from coral reefs and tropical latitudes (29). Although adult body size is not significantly associated with such gaps, small body size correlates significantly with reduced larval dispersal and small geographic ranges and can be used as an indicator of extinction risk (28, 29).
Speciation also is related to life history characteristics and geographic range size. Although the relationship between geographic range size and speciation has been debated (36, 75, 76, 82, 90), range size is positively related to dispersal ability and gene flow in many marine and terrestrial taxa (12, 28, 29, 59, 66, 91), and these factors tend to dampen rates of speciation (66, 72, 92). Paleontological approaches allow measurement of both speciation rates and geographic range sizes over time. Range size is significantly inversely related to speciation rates in fossil gastropods and brachiopods (66, 82, 85, 93).
Because it constrains reproductive traits and geographic range, body size can be used as an indicator of speciation rate. Evidence that small-bodied species are engines of diversification comes from studies of morphological and taxonomic divergence of stomatopod lineages across biogeographic barriers (12, 29). Large-bodied lineages and those with long-lived larvae remain conspecific (morphologically indistinguishable) or closely related (cognate species) when separated by a barrier (e.g., the Central American isthmus) significantly more frequently than small-bodied species with abbreviated larval development. Conversely, the percentage of endemic species without cognates elsewhere and the percentage of species within locally radiating clusters of species (more closely related to each other than to any species outside the region) are significantly elevated in lineages of small body size, of limited larval dispersal, and from reefs.
Recent molecular studies, including those on gastropods and small-bodied stomatopods, show that incipient or full speciation is more common in reef fauna than previously thought (24, 94–97). In addition to the fact that most benthic reef species are small in body size (97–99) with restricted or moderate colonizing ability, the behavior of reef larvae further reduces dispersal. Stomatopod larvae (and those of most other reef taxa) exhibit diurnal vertical migrations, hiding in reef rubble by day and migrating into the water column at dusk and dawn (100), which reduces exposure to currents. Although Panda clownfish have a 9- to 12-day pelagic phase, one-third of marked juveniles settle within their natal area, many within 100 m of their birth site (101).
Discussion
Using body size as an indicator of speciation and extinction rates, we infer that the IAA, and to a lesser extent the IOC, are areas of both high origination and high extinction in reef stomatopods. However, rates of origination must exceed those of extinction in these areas, yielding the high biodiversity observed. Endemism results from either newly originated or almost extinct species and thus is expected to be especially high if both speciation and extinction are elevated, as is observed. Although species are concentrated in small size classes in the IAA and IOC, the range of body sizes is large in these areas (see Fig. S3). Historical factors (faunal carryover from the Tethyan Seaway), productivity in the continental areas, currents, and species diversity itself (via ecological interactions between species) likely have contributed to the species richness and range of body sizes in the IOC and IAA. The dispersal and colonizing capability of large-sized species in these areas allows them to disproportionally colonize adjacent oceanic regions, where extensive larval immigration lowers extinction and retards speciation, yielding moderately diverse, somewhat larger-sized assemblages with low endemism.
In the center of the IO and in the broader expanse of the Pacific, however, larval immigrants have been filtered by starvation, predation, and distance. Given enough time, it is likely that larvae from diversity centers reach mid-ocean islands. However, both diversity and adult body size of reef stomatopods decline in the mid-Pacific, and body size is smaller on mid-Pacific atolls than on high islands, suggesting that productivity of the island environment, as well as propagule pressure, influences successful colonization. Dwarfed by limited productivity, populations cannot produce sufficient propagules to reach another island archipelago and are unlikely to receive many immigrants from ancestral populations to the west. They diverge into new species; endemism increases toward the CP. However, extinction also must be exceedingly high in these small-sized peripheral species. Endemics are missing from atolls, probably reflecting the difficulty of establishing successful populations in these low-productivity environments that are heavily dominated by top predators (M.L.R., personal observation). We have observed one instance of population extinction in a small-sized reef stomatopod from a mid-Pacific atoll (102). Consequently, the wheels of speciation and extinction turn rapidly, but in reverse direction. If species arrive, speciation is high but extinction even higher; thus, diversity is low in remote oceanic regions of the IO and CP. Although the available evidence from life histories, geographic ranges, and extinction/speciation in stomatopods and other organisms supports this interpretation, molecular evidence on ages of species also is needed.
We conclude that life history patterns and dispersal are the primary mediators of the rate and direction of the speciation/extinction cycle, which in turn determines the geography of diversity and endemism. However, productivity, historical factors (antecedent faunas), and currents likely influence diversity in particular localities. In addition, productivity, historical factors (lineage history), and species diversity itself (through ecological interactions) alter body size and thus influence life history and dispersal.
Acknowledgments.
We thank F. J. Ayala and J. C. Avise and two reviewers for helpful comments that improved the article.
Footnotes
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “In the Light of Evolution II: Biodiversity and Extinction,” held December 6–8, 2007, at the Arnold and Mabel Beckman Center of the National Academies of Sciences and Engineering in Irvine, CA. The complete program and audio files of most presentations are available on the NAS web site at www.nasonline.org/Sackler_biodiversity.
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0802594105/DCSupplemental.
References
- 1.Bryant D, Burke L, McManus J, Spalding M. Reefs at Risk, A Map-Based Indicator of Threats to the World's Coral Reefs. Washington, DC: World Resources Institute; 1998. pp. 1–56. [Google Scholar]
- 2.Burke L, Selig E, Spalding M. Reefs at Risk in Southeast Asia. Washington, DC: World Resources Institute; 2002. pp. 1–72. [Google Scholar]
- 3.Gardner TA, Cote IM, Gill JA, Grant A, Watkinson AR. Long-term region-wide declines in Caribbean corals. Science. 2003;301:958–960. doi: 10.1126/science.1086050. [DOI] [PubMed] [Google Scholar]
- 4.Hughes TP, et al. Climate change, human impacts, and the resilience of coral reefs. Science. 2003;301:929–933. doi: 10.1126/science.1085046. [DOI] [PubMed] [Google Scholar]
- 5.Pandolfi JM, et al. Global trajectories of the long-term decline of coral reef ecosystems. Science. 2003;301:955–958. doi: 10.1126/science.1085706. [DOI] [PubMed] [Google Scholar]
- 6.Bellwood DR, Hughes TP, Folk C, Nystrom M. Confronting the coral reef crisis. Nature. 2004;429:827–833. doi: 10.1038/nature02691. [DOI] [PubMed] [Google Scholar]
- 7.Burke L, Maidens J. Reefs at Risk in the Caribbean. Washington, DC: World Resources Institute; 2004. pp. 1–80. [Google Scholar]
- 8.Pandolfi JM, et al. Are US coral reefs on the slippery slope to slime? Science. 2005;307:1725–1726. doi: 10.1126/science.1104258. [DOI] [PubMed] [Google Scholar]
- 9.Hoegh-Guldberg O, et al. Coral reefs under rapid climate change and ocean acidification. Science. 2007;318:1737–1742. doi: 10.1126/science.1152509. [DOI] [PubMed] [Google Scholar]
- 10.Wilkinson C, editor. Status of the Coral Reefs of the World. Vols 1 and 2. Townsville, Australia: Australian Inst Mar Sci; 2004. [Google Scholar]
- 11.Caldwell RL, Dingle H. Ecology and evolution of agonistic behavior in stomatopods. Naturwissenschaften. 1975;62:214–222. [Google Scholar]
- 12.Reaka ML, Manning RB. The behavior of stomatopod Crustacea, and its relationship to rates of evolution. J Crust Biol. 1981;1:309–327. [Google Scholar]
- 13.Steli FG, Wells JW. Diversity and age patterns in hermatypic corals. Syst Zool. 1971;20:115–126. [Google Scholar]
- 14.Paulay G. In: Life and Death of Coral Reefs. Birkeland C, editor. New York: Chapman & Hall; 1997. pp. 298–353. [Google Scholar]
- 15.Briggs JC. Global Biogeography. Amsterdam: Elsevier; 1995. [Google Scholar]
- 16.Briggs JC. Coincident biogeographic patterns: Indo-West Pacific Ocean. Evolution (Lawrence, Kans) 1999;53:326–335. doi: 10.1111/j.1558-5646.1999.tb03769.x. [DOI] [PubMed] [Google Scholar]
- 17.Briggs JC. Centrifugal speciation and centres of origin. J Biogeogr. 2000;27:1183–1188. [Google Scholar]
- 18.Briggs JC. Marine centres of origin as evolutionary engines. J Biogeogr. 2003;30:1–18. [Google Scholar]
- 19.Briggs JC. Marine longitudinal biodiversity: Causes and conservation. Diversity Distrib. 2007;13:544–555. [Google Scholar]
- 20.Veron JE. Corals in Space and Time: The Biogeography and Evolution of the Scleractinia. Sydney, Australia: Univ of New South Wales Press; 1995. [Google Scholar]
- 21.Bellwood DR, Hughes TP. Regional-scale assembly rules and biodiversity of coral reefs. Science. 2001;292:1532–1534. doi: 10.1126/science.1058635. [DOI] [PubMed] [Google Scholar]
- 22.Hughes TP, Bellwood DR, Connolly SR. Biodiversity hotspots, centers of endemicity, and the conservation of coral reefs. Ecol Lett. 2002;5:775–784. [Google Scholar]
- 23.Roberts CM, et al. Marine biodiversity hotspots and conservation priorities for tropical reefs. Science. 2002;295:1280–1284. doi: 10.1126/science.1067728. [DOI] [PubMed] [Google Scholar]
- 24.Meyer CP. Molecular systematics of cowries (Gastropoda: Cypraeidae) and diversification patterns in the tropics. Biol J Linn Soc. 2003;79:401–459. [Google Scholar]
- 25.Mora C, Chittaro PM, Sale PF, Kritzer JP, Ludsin SA. Patterns and processes in reef fish diversity. Nature. 2003;421:933–936. doi: 10.1038/nature01393. [DOI] [PubMed] [Google Scholar]
- 26.Bellwood DR, Hughes TP, Connolly SR, Tanner J. Environmental and geometric constraints on Indo-Pacific coral reef biodiversity. Ecol Lett. 2005;8:643–651. [Google Scholar]
- 27.Reaka ML. In: Reproductive Ecology of Marine Invertebrates. Stancyk S, editor. Columbia: Univ of South Carolina Press; 1979. pp. 235–260. [Google Scholar]
- 28.Reaka ML. Geographic range, life history patterns, and body size in a guild of coral-dwelling mantis shrimps. Evolution (Lawrence, Kans) 1980;34:1019–1030. doi: 10.1111/j.1558-5646.1980.tb04041.x. [DOI] [PubMed] [Google Scholar]
- 29.Reaka ML, Manning RB. The significance of body size, dispersal potential, and habitat for rates of morphological evolution in stomatopod Crustacea. Smithson Contrib Zool. 1987;448:1–45. [Google Scholar]
- 30.Ahyong ST. Revision of the Australian Stomatopod Crustacea. Rec Aust Mus (Suppl) 2001;26:1–326. [Google Scholar]
- 31.Schram FR, Muller H. Catalog and Bibliography of the Fossil and Recent Stomatopoda. Leiden, The Netherlands: Backhuys; 2004. [Google Scholar]
- 32.Ladd HS. Origin of the Pacific island molluscan fauna. Am J Sci. 1960;258A:137–150. [Google Scholar]
- 33.Jokiel P, Martinelli FJ. The vortex model of coral reef biogeography. J Biogeogr. 1992;19:449–458. [Google Scholar]
- 34.Connolly SR, Bellwood DR, Hughes TP. Indo-Pacific biodiversity of coral reefs: Deviations from a mid-domain model. Ecology. 2003;84:2178–2190. [Google Scholar]
- 35.Barber PH, Bellwood DR. Biodiversity hotspots: Evolutionary origins of biodiversity in wrasses (Halichoeres: Labridae) in the Indo-Pacific and new world tropics. Mol Phylogenet Evol. 2005;35:235–253. doi: 10.1016/j.ympev.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 36.Rosenzweig ML. Species Diversity in Space and Time. Cambridge, U.K.: Cambridge Univ Press; 1995. [Google Scholar]
- 37.Allen AP, Gillooly JF, Savage VM, Brown JH. Kinetic effects of temperature on rates of genetic divergence and speciation. Proc Natl Acad Sci USA. 2006;103:9130–9135. doi: 10.1073/pnas.0603587103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Allen AP, Gillooly JF. Assessing latitudinal gradients in speciation rates and biodiversity at the global scale. Ecol Lett. 2006;9:947–954. doi: 10.1111/j.1461-0248.2006.00946.x. [DOI] [PubMed] [Google Scholar]
- 39.Birkeland C. Terrestrial runoff as a cause of outbreaks of Acanthaster planci (Echinodermata: Asteroidea) Mar Biol. 1982;69:175–185. [Google Scholar]
- 40.Brodie J, Fabricius K, De'ath G, Okaji K. Are increased nutrient inputs responsible for more outbreaks of crown-of-thorns starfish? An appraisal of the evidence. Mar Poll Bull. 2005;51:266–278. doi: 10.1016/j.marpolbul.2004.10.035. [DOI] [PubMed] [Google Scholar]
- 41.Houk P, Bograd S, van Woesik R. The transition zone chlorophyll front can trigger Acanthaster planci outbreaks in the Pacific Ocean: Historical confirmation. J Oceanogr. 2007;63:149–154. [Google Scholar]
- 42.Briggs JC. Extinction and replacement in the Indo-West Pacific Ocean. J Biogeogr. 1999;26:777–783. [Google Scholar]
- 43.Emerson BC, Kolm N. Species diversity can drive speciation. Nature. 2005;434:1015–1017. doi: 10.1038/nature03450. [DOI] [PubMed] [Google Scholar]
- 44.Cardena CD, Ricklefs RR, Jimenez I, Bermingham E. Is speciation driven by species diversity? Nature. 2005;438:E1–E2. doi: 10.1038/nature04308. [DOI] [PubMed] [Google Scholar]
- 45.Myers N. Threatened biotas: “Hotspots” in tropical forests. Environmentalist. 1988;8:187–208. doi: 10.1007/BF02240252. [DOI] [PubMed] [Google Scholar]
- 46.Reid WV. Biodiversity hotspots. Trends Ecol Evol. 1998;13:275–280. doi: 10.1016/s0169-5347(98)01363-9. [DOI] [PubMed] [Google Scholar]
- 47.Myers N, Mittermeier RA, Mittermeier CG, da Fonseca G, Kent J. Biodiversity hotspots for conservation priorities. Nature. 2000;403:853–858. doi: 10.1038/35002501. [DOI] [PubMed] [Google Scholar]
- 48.Mittermeier RA, et al. Hotspots Revisited: Earth's Biologically Richest and Most Endangered Terrestrial Ecoregions. Mexico City, Mexico: CEMEX/Agrupación Sierra Madre; 2004. [Google Scholar]
- 49.Prendergast JR, Quinn RM, Lawton JH, Eversham BC, Gibbons DW. Rare species, the coincidence of diversity hotspots and conservation strategies. Nature. 1993;365:335–337. [Google Scholar]
- 50.Williams P, et al. A comparison of richness hotspots, rarity hotspots, and complementary areas for conserving diversity of British birds. Conserv Biol. 1996;10:155–174. [Google Scholar]
- 51.Bonn A, Rodriguez ASL, Gaston KJ. Threatened and endemic species: Are they good indicators of patterns of biodiversity on a national scale? Ecol Lett. 2002;5:733–741. [Google Scholar]
- 52.Orme CDL, et al. Global hotspots of species richness are not congruent with endemism or threat. Nature. 2005;436:1016–1019. doi: 10.1038/nature03850. [DOI] [PubMed] [Google Scholar]
- 53.Baird AH, et al. Coral reef biodiversity and conservation. Science. 2002;296:1026–1028. doi: 10.1126/science.296.5570.1026. [DOI] [PubMed] [Google Scholar]
- 54.Briggs JC. Coral reef biodiversity and conservation. Science. 2002;296:1026–1028. [PubMed] [Google Scholar]
- 55.Reaka-Kudla ML. The evolution of endemism in insular Pacific faunas: Coral-dwelling stomatopods. J Crust Biol. 2000;20:56–70. [Google Scholar]
- 56.Reaka-Kudla ML. In: The Unity of Evolutionary Biology. Dudley EC, editor. Vol 1. Portland, OR: Dioscorides Press; 1991. pp. 61–70. [Google Scholar]
- 57.Menge BA. Brood or broadcast? The adaptive significance of different reproductive strategies in two intertidal sea stars Leptasterias hexactis and Pisaster ochraceus. Mar Biol. 1975;31:87–100. [Google Scholar]
- 58.Strathmann RR, Strathmann MF. The relationship between adult size and brooding in marine invertebrates. Am Nat. 1982;119:91–101. [Google Scholar]
- 59.Jablonski D, Lutz RA. Larval ecology of marine benthic invertebrates: Paleobiological implications. Biol Rev. 1983;58:21–89. [Google Scholar]
- 60.Jablonski D. Larval ecology and macroevolution of marine invertebrates. Bull Mar Sci. 1986;39:565–587. [Google Scholar]
- 61.Jablonski D. In: Evolutionary Paleobiology. Jablonski D, Erwin DH, Lipps JH, editors. Chicago: Univ Chicago Press; 1996. pp. 256–289. [Google Scholar]
- 62.Strathmann RR. Feeding and nonfeeding larval development and life history evolution in marine invertebrates. Annu Rev Ecol Syst. 1985;16:339–361. [Google Scholar]
- 63.Strathmann RR. Why life histories evolve differently in the sea. Amer Zool. 1990;30:197–207. [Google Scholar]
- 64.Roy K, Jablonski D, Valentine JW. Climate change, species range limits and body size in marine bivalves. Ecol Lett. 2001;4:366–370. [Google Scholar]
- 65.Roy K, Jablonski D, Valentine JW. Body size and invasion success in marine bivalves. Ecol Lett. 2002;5:163–167. [Google Scholar]
- 66.Jablonski D, Roy K, Valentine JW. In: Macroecology: Concepts and Consequences. Blackburn TM, Gaston KJ, editors. Oxford: Blackwell; 2003. pp. 368–390. [Google Scholar]
- 67.May RM. In: Diversity of Insect Faunas. Mound LA, Waloff N, editors. Oxford: Blackwell; 1978. pp. 188–204. [Google Scholar]
- 68.May RM. The search for patterns in the balance of nature: Advances and retreats. Ecology. 1986;67:1115–1126. [Google Scholar]
- 69.May RM. How many species are there on Earth? Science. 1988;241:1441–1449. doi: 10.1126/science.241.4872.1441. [DOI] [PubMed] [Google Scholar]
- 70.Gaston KJ, Blackburn TM. Pattern and Process in Macroecology. Oxford: Blackwell; 2000. [Google Scholar]
- 71.Roy K, Jablonski D, Martien KK. Invariant size-frequency distributions along a latitudinal gradient in marine bivalves. Proc Natl Acad Sci USA. 2000;97:13150–13155. doi: 10.1073/pnas.97.24.13150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hubbell SP. The Unified Neutral Theory of Biodiversity and Biogeography. Princeton: Princeton Univ Press; 2001. [DOI] [PubMed] [Google Scholar]
- 73.Gaston KJ. Rarity. London: Chapman and Hall; 1994. [Google Scholar]
- 74.Gaston KJ, Blackburn TM. Conservation implications of geographic range size–body size relationships. Conserv Biol. 1996;10:638–646. [Google Scholar]
- 75.Gaston KJ. Species-range size distributions: Products of speciation, extinction and transformation. Philos Trans R Soc London B. 1998;353:219–230. [Google Scholar]
- 76.Gaston KJ, Chown SL. In: Evolution of Biological Diversity. Magurran AE, May RM, editors. Oxford: Oxford Univ Press; 1999. pp. 236–259. [Google Scholar]
- 77.Roberts CM, Hawkins JP. Extinction risk in the sea. Trends Ecol Evol. 1999;14:241–236. doi: 10.1016/s0169-5347(98)01584-5. [DOI] [PubMed] [Google Scholar]
- 78.Jablonski D. Background and mass extinctions: The alternation of macroevolutionary regimes. Science. 1986;231:129–133. doi: 10.1126/science.231.4734.129. [DOI] [PubMed] [Google Scholar]
- 79.Jablonski D. Heritability at the species level: Analysis of geographic ranges of Cretaceous molluscs. Science. 1987;238:360–363. doi: 10.1126/science.238.4825.360. [DOI] [PubMed] [Google Scholar]
- 80.Jablonski D. Extinctions: A paleontological perspective. Science. 1991;253:754–757. doi: 10.1126/science.253.5021.754. [DOI] [PubMed] [Google Scholar]
- 81.Jablonski D, Hunt E. Larval ecology, geographic range, and species survivorship in Cretaceous mollusks: Organismic vs. species-level explanations. Am Nat. 2006;168:556–564. doi: 10.1086/507994. [DOI] [PubMed] [Google Scholar]
- 82.Jablonski D. Scale and hierarchy in macroevolution. Palaeontology. 2007;50:87–109. [Google Scholar]
- 83.Powell MG. Geographic range and genus longevity of late Paleozoic brachiopods. Paleobiology. 2007;33:530–346. [Google Scholar]
- 84.Jablonski D. The tropics as a source of evolutionary novelty through geological time. Nature. 1993;364:142–144. [Google Scholar]
- 85.Powell MG. Latitudinal diversity gradients for brachiopod genera during late Palaeozoic time: Links between climate, biogeography and evolutionary rates. Global Ecol Biogeogr. 2007;16:519–528. [Google Scholar]
- 86.Jackson JBC, Budd AF, Coates AG, editors. Evolution and Environment in Tropical America. Chicago: Univ Chicago Press; 1996. [Google Scholar]
- 87.Budd AF. Diversity and extinction in the Cenozoic history of Caribbean reefs. Coral Reefs. 2000;19:25–35. [Google Scholar]
- 88.Jackson JBC, Johnson KG. Life in the last few million years. Paleobiology. 2000;26:221–235. [Google Scholar]
- 89.Todd JA, et al. The ecology of extinction: Molluscan feeding and faunal turnover in the Caribbean Neogene. Proc R Soc London Ser B. 2002;269:571–577. doi: 10.1098/rspb.2001.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chown SL. In: The Biology of Rarity. Kunin WE, Gaston KJ, editors. London: Chapman & Hall; 1997. pp. 91–109. [Google Scholar]
- 91.Paulay G, Meyer C. Dispersal and divergence across the greatest ocean region: Do larvae matter? Integr Comp Biol. 2006;46:269–281. doi: 10.1093/icb/icj027. [DOI] [PubMed] [Google Scholar]
- 92.Mayr E. Animal Species and Evolution. Cambridge, MA: Harvard Univ Press; 1963. [Google Scholar]
- 93.Jablonski D, Roy K. Geographic range and speciation in fossil and living molluscs. Proc R Soc London Ser B. 2003;270:401–406. doi: 10.1098/rspb.2002.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Barber PH, Palumbi SR, Erdmann MV, Moosa MK. A marine Wallace's Line? Nature. 2000;406:692–693. doi: 10.1038/35021135. [DOI] [PubMed] [Google Scholar]
- 95.Barber PH, Palumbi SR, Erdmann MV, Moosa MK. Sharp genetic breaks among populations of a benthic marine crustaceans indicate limited oceanic larval transport: Patterns, causes, and consequences. Mol Ecol. 2002;11:659–674. doi: 10.1046/j.1365-294x.2002.01468.x. [DOI] [PubMed] [Google Scholar]
- 96.Barber PH, Erdmann MV, Palumbi SR. Comparative phylogeography of three codistributed stomatopods: Origins and timing of regional lineage diversification in the coral triangle. Evolution (Lawrence, Kans) 2006;60:1825–1839. [PubMed] [Google Scholar]
- 97.Meyer CP, Geller JP, Paulay G. Fine-scale endemism on coral reefs: Archipelagic differentiation in turbinid gastropods. Evolution (Lawrence, Kans) 2005;59:113–125. [PubMed] [Google Scholar]
- 98.Reaka-Kudla ML. In: Biodiversity II: Understanding and Protecting Our Natural Resources. Reaka-Kudla ML, Wilson DE, Wilson EO, editors. Washington, DC: Joseph Henry Press; 1997. pp. 83–108. [Google Scholar]
- 99.Bouchet P, Lozouet P, Maestrati P, Heros V. Assessing the magnitude of species richness in tropical marine environments: Exceptionally high numbers of molluscs at a New Caledonia site. Biol J Linn Soc. 2002;75:421–436. [Google Scholar]
- 100.Robichaux DM, Cohen AC, Reaka ML, Allen D. Experiments with zooplankton on coral reefs, or, will the real demersal plankton please come up? Mar Ecol. 1981;2:77–94. [Google Scholar]
- 101.Jones GP, Planes S, Thorrold SR. Coral reef fish larvae settle close to home. Curr Biol. 2005;15:1314–1318. doi: 10.1016/j.cub.2005.06.061. [DOI] [PubMed] [Google Scholar]
- 102.Reaka ML, Manning RB. In: The Natural History of Enewetak Atoll Vol. 2, Biogeography and Systematics. Deveney DM, Reese ES, Burch BL, Helfrich P, editors. Washington, DC: Office of Scientific and Technical Information, US Dept of Energy; 1987. pp. 181–190. [Google Scholar]