Abstract
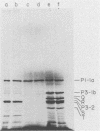
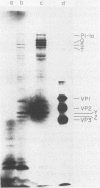
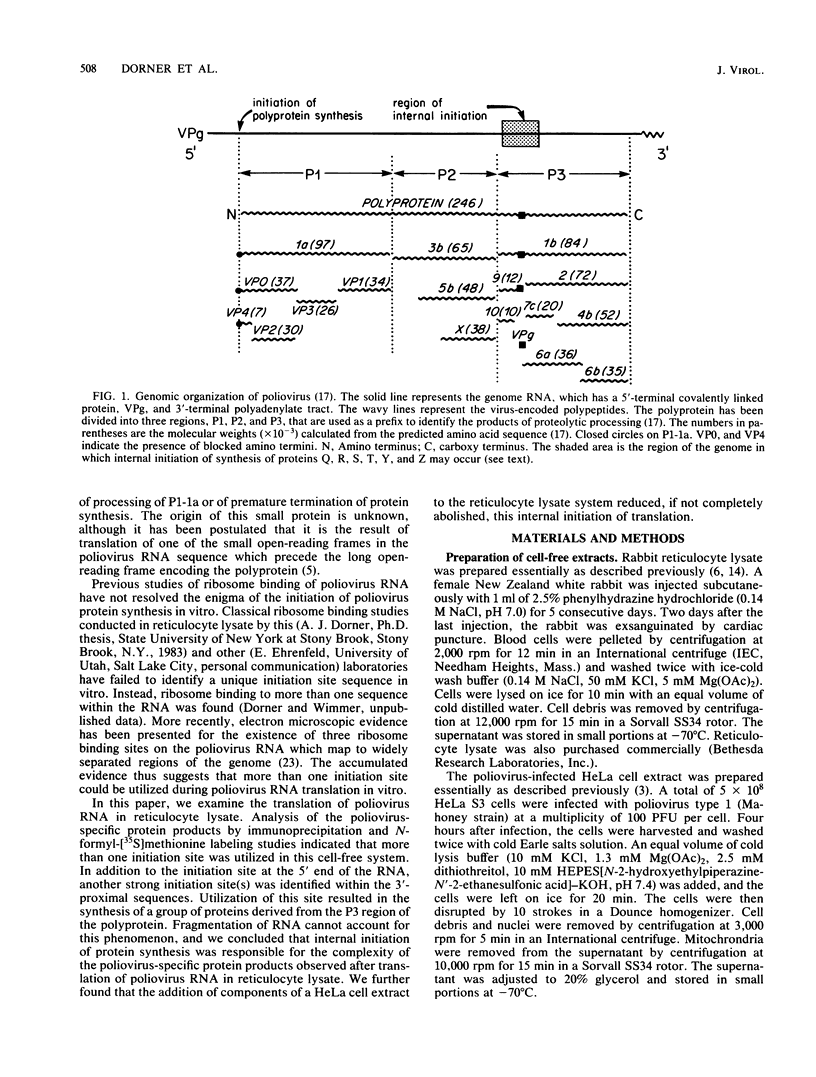
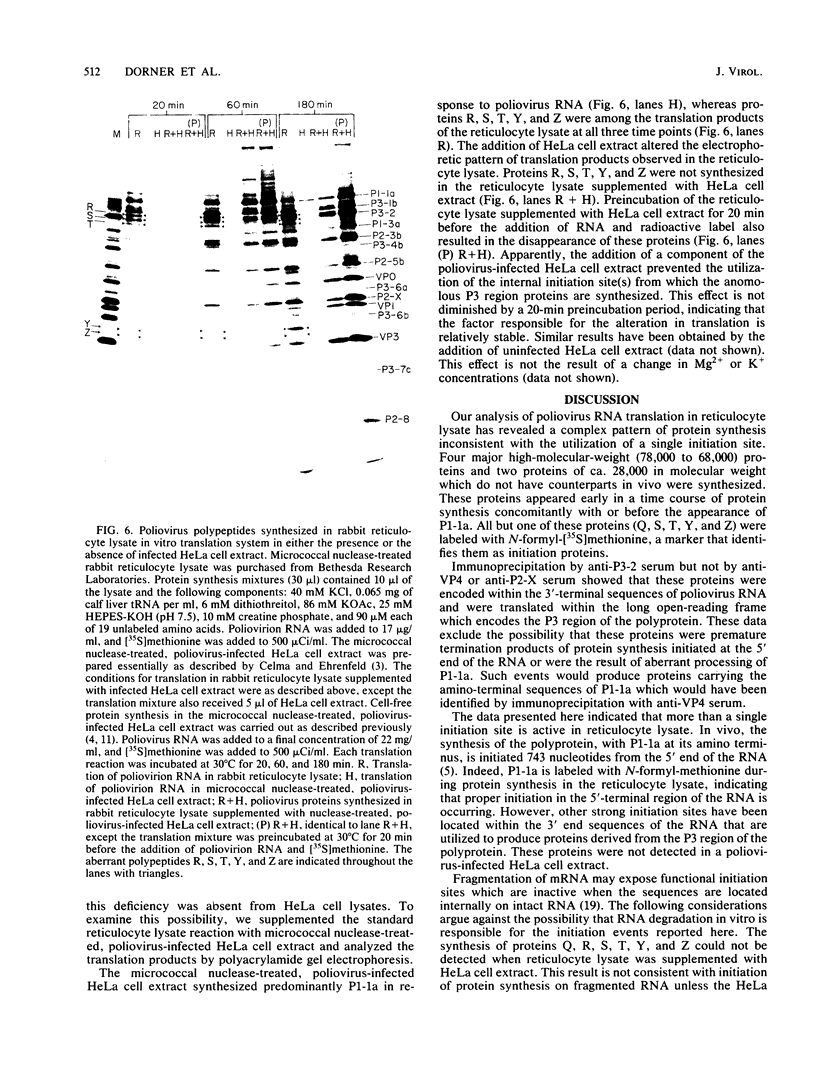
The translation of poliovirus RNA in rabbit reticulocyte lysate was examined. Translation of poliovirus RNA in this cell-free system resulted in an electrophoretic profile of poliovirus-specific proteins distinct from that observed in vivo or after translation in poliovirus-infected HeLa cell extract. A group of proteins derived from the P3 region of the polyprotein was identified by immunoprecipitation, time course, and N-formyl-[35S]methionine labeling studies to be the product of the initiation of protein synthesis at an internal site(s) located within the 3'-proximal RNA sequences. Utilization of this internal initiation site(s) on poliovirus RNA was abolished when reticulocyte lysate was supplemented with poliovirus-infected HeLa cell extract. Authentic P1-1a was also synthesized in reticulocyte lysate, indicating that correct 5'-proximal initiation of translation occurs in that system. We conclude that the deficiency of a component(s) of the reticulocyte lysate necessary for 5'-proximal initiation of poliovirus protein synthesis resulted in the ability of ribosomes to initiate translation on internal sequences. This aberrant initiation could be corrected by factors present in the HeLa cell extract. Apparently, under certain conditions, ribosomes are capable of recognizing internal sequences as authentic initiation sites.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkins J. F., Steitz J. A., Anderson C. W., Model P. Binding of mammalian ribosomes to MS2 phage RNA reveals an overlapping gene encoding a lysis function. Cell. 1979 Oct;18(2):247–256. doi: 10.1016/0092-8674(79)90044-8. [DOI] [PubMed] [Google Scholar]
- Brown B. A., Ehrenfeld E. Translation of poliovirus RNA in vitro: changes in cleavage pattern and initiation sites by ribosomal salt wash. Virology. 1979 Sep;97(2):396–405. doi: 10.1016/0042-6822(79)90350-7. [DOI] [PubMed] [Google Scholar]
- Celma M. L., Ehrenfeld E. Translation of poliovirus RNA in vitro: detection of two different initiation sites. J Mol Biol. 1975 Nov 15;98(4):761–780. doi: 10.1016/s0022-2836(75)80009-x. [DOI] [PubMed] [Google Scholar]
- Daniels-McQueen S., Detjen B. M., Grifo J. A., Merrick W. C., Thach R. E. Unusual requirements for optimum translation of polio viral RNA in vitro. J Biol Chem. 1983 Jun 10;258(11):7195–7199. [PubMed] [Google Scholar]
- Dorner A. J., Dorner L. F., Larsen G. R., Wimmer E., Anderson C. W. Identification of the initiation site of poliovirus polyprotein synthesis. J Virol. 1982 Jun;42(3):1017–1028. doi: 10.1128/jvi.42.3.1017-1028.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorner A. J., Kemper B. Conversion of pre-proparathyroid hormone to proparathyroid hormone by dog pancreatic microsomes. Biochemistry. 1978 Dec 12;17(25):5550–5555. doi: 10.1021/bi00618a034. [DOI] [PubMed] [Google Scholar]
- Dorner A. J., Rothberg P. G., Wimmer E. The fate of VPg during in vitro translation of poliovirus RNA. FEBS Lett. 1981 Sep 28;132(2):219–223. doi: 10.1016/0014-5793(81)81164-7. [DOI] [PubMed] [Google Scholar]
- Ehrenfeld E., Brown D. Stability of poliovirus RNA in cell-free translation systems utilizing two initiation sites. J Biol Chem. 1981 Mar 25;256(6):2656–2661. [PubMed] [Google Scholar]
- Emini E. A., Dorner A. J., Dorner L. F., Jameson B. A., Wimmer E. Identification of a poliovirus neutralization epitope through use of neutralizing antiserum raised against a purified viral structural protein. Virology. 1983 Jan 15;124(1):144–151. doi: 10.1016/0042-6822(83)90297-0. [DOI] [PubMed] [Google Scholar]
- Emini E. A., Elzinga M., Wimmer E. Carboxy-terminal analysis of poliovirus proteins: termination of poliovirus RNA translation and location of unique poliovirus polyprotein cleavage sites. J Virol. 1982 Apr;42(1):194–199. doi: 10.1128/jvi.42.1.194-199.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanecak R., Semler B. L., Anderson C. W., Wimmer E. Proteolytic processing of poliovirus polypeptides: antibodies to polypeptide P3-7c inhibit cleavage at glutamine-glycine pairs. Proc Natl Acad Sci U S A. 1982 Jul;79(13):3973–3977. doi: 10.1073/pnas.79.13.3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries S., Knauert F., Ehrenfeld E. Capsid protein precursor is one of two initiated products of translation of poliovirus RNA in vitro. J Virol. 1979 May;30(2):481–488. doi: 10.1128/jvi.30.2.481-488.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson R. J., Hunt T. Preparation and use of nuclease-treated rabbit reticulocyte lysates for the translation of eukaryotic messenger RNA. Methods Enzymol. 1983;96:50–74. doi: 10.1016/s0076-6879(83)96008-1. [DOI] [PubMed] [Google Scholar]
- Jacobson M. F., Asso J., Baltimore D. Further evidence on the formation of poliovirus proteins. J Mol Biol. 1970 May 14;49(3):657–669. doi: 10.1016/0022-2836(70)90289-5. [DOI] [PubMed] [Google Scholar]
- Jacobson M. F., Baltimore D. Polypeptide cleavages in the formation of poliovirus proteins. Proc Natl Acad Sci U S A. 1968 Sep;61(1):77–84. doi: 10.1073/pnas.61.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura N., Semler B. L., Rothberg P. G., Larsen G. R., Adler C. J., Dorner A. J., Emini E. A., Hanecak R., Lee J. J., van der Werf S. Primary structure, gene organization and polypeptide expression of poliovirus RNA. Nature. 1981 Jun 18;291(5816):547–553. doi: 10.1038/291547a0. [DOI] [PubMed] [Google Scholar]
- Kozak M. Binding of wheat germ ribosomes to fragmented viral mRNA. J Virol. 1980 Sep;35(3):748–756. doi: 10.1128/jvi.35.3.748-756.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Comparison of initiation of protein synthesis in procaryotes, eucaryotes, and organelles. Microbiol Rev. 1983 Mar;47(1):1–45. doi: 10.1128/mr.47.1.1-45.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larsen G. R., Anderson C. W., Dorner A. J., Semler B. L., Wimmer E. Cleavage sites within the poliovirus capsid protein precursors. J Virol. 1982 Jan;41(1):340–344. doi: 10.1128/jvi.41.1.340-344.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClain K., Stewart M., Sullivan M., Maizel J. V., Jr Ribosomal binding sites on poliovirus RNA. Virology. 1981 Aug;113(1):150–167. doi: 10.1016/0042-6822(81)90144-6. [DOI] [PubMed] [Google Scholar]
- Rekosh D. Gene order of the poliovirus capsid proteins. J Virol. 1972 Mar;9(3):479–487. doi: 10.1128/jvi.9.3.479-487.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semler B. L., Anderson C. W., Kitamura N., Rothberg P. G., Wishart W. L., Wimmer E. Poliovirus replication proteins: RNA sequence encoding P3-1b and the sites of proteolytic processing. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3464–3468. doi: 10.1073/pnas.78.6.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semler B. L., Hanecak R., Anderson C. W., Wimmer E. Cleavage sites in the polypeptide precursors of poliovirus protein P2-X. Virology. 1981 Oct 30;114(2):589–594. doi: 10.1016/0042-6822(81)90242-7. [DOI] [PubMed] [Google Scholar]
- Semler B. L., Hanecak R., Dorner L. F., Anderson C. W., Wimmer E. Poliovirus RNA synthesis in vitro: structural elements and antibody inhibition. Virology. 1983 Apr 30;126(2):624–635. doi: 10.1016/s0042-6822(83)80018-x. [DOI] [PubMed] [Google Scholar]
- Shih D. S., Shih C. T., Kew O., Pallansch M., Rueckert R., Kaesberg P. Cell-free synthesis and processing of the proteins of poliovirus. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5807–5811. doi: 10.1073/pnas.75.12.5807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers D. F., Maizel J. V., Jr Determination of the gene sequence of poliovirus with pactamycin. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2852–2856. doi: 10.1073/pnas.68.11.2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taber R., Rekosh D., Baltimore D. Effect of pactamycin on synthesis of poliovirus proteins: a method for genetic mapping. J Virol. 1971 Oct;8(4):395–401. doi: 10.1128/jvi.8.4.395-401.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P., Andersen B. Partial purification of SV40 A protein and a related cellular protein from permissive cells. Virology. 1981 Nov;115(1):67–74. doi: 10.1016/0042-6822(81)90089-1. [DOI] [PubMed] [Google Scholar]
- Villa-Komaroff L., Guttman N., Baltimore D., Lodishi H. F. Complete translation of poliovirus RNA in a eukaryotic cell-free system. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4157–4161. doi: 10.1073/pnas.72.10.4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARNER J., MADDEN M. J., DARNELL J. E. The interaction of poliovirus RNA with Escherichia coli ribosomes. Virology. 1963 Mar;19:393–399. doi: 10.1016/0042-6822(63)90079-5. [DOI] [PubMed] [Google Scholar]