Abstract
The anatomy of strawberry (Fragaria × ananassa) fruit, in which the achene is found on the outer part of the fruit, makes it an excellent species for studying the regulation of fruit development. It can provide a model for the cross talk between primary and secondary metabolism, whose role is of pivotal importance in the process. By combining gas chromatography-mass spectrometry and liquid chromatography-mass spectrometry with the aim of addressing the metabolic regulation underlying fruit seed development, we simultaneously analyzed the composition of primary and secondary metabolites, separately, in achene and receptacle during fruit ripening of strawberry cultivar Herut. The results from these analyses suggest that changes in primary and secondary metabolism reflect organ and developmental specificities. For instance, the receptacle was characterized by increases in sugars and their direct derivatives, while the achene was characterized by a major decrease in the levels of carbon- and nitrogen-rich compounds, with the exception of storage-related metabolites (e.g. raffinose). Furthermore, the receptacle, and to a lesser extent the achene, exhibited dynamic fluctuations in the levels and nature of secondary metabolites across the ripening process. In the receptacle, proanthocyanidins and flavonol derivatives characterized mainly early developmental stages, while anthocyanins were abundant in the mature red stage; in the achene, ellagitannin and flavonoids were abundant during early and late development, respectively. Correlation-based network analysis suggested that metabolism is substantially coordinated during early development in either organ. Nonetheless, a higher degree of connectivity within and between metabolic pathways was measured in the achenes. The data are discussed within the context of current models both of the interaction of primary and secondary metabolism and of the metabolic interaction between the different plant organs.
In many plants, fruit development can be divided into four distinct phases (Gillaspy et al., 1993). The first phase commences after flower opening (anthesis) and involves fertilization and development of the ovary (in so-called true fruit) and is generally referred to as fruit set. In the second phase, fruit growth by cell division is the most prominent process and is accompanied by seed and early embryo formation. In the third phase, following cell division, fruit growth is maintained largely by increases in cell volume. During this stage of fruit expansion, the embryo passes through a maturation phase. This phase often leads to the induction of seed dormancy and is characterized by (1) the accumulation of storage products, (2) the suppression of precocious germination, (3) the acquisition of desiccation tolerance, and (4) water loss (Bewley and Black, 1994).
Strawberry (Fragaria × ananassa) fruit initiate from a single inflorescence and are actually an aggregate, composed of many ovaries, each with a single ovule (Perkins-Veazie, 1995). The seeds (achenes), embedded in the epidermis of the swollen receptacle, are the true fruit of this species. The receptacle is composed of an internal pith, a cortex layer, and an epidermal layer (Suutarinen et al., 1998). Fibrovascular strands connect the achenes to the interior of the receptacle, and they supply nutrients to the achenes and the surrounding parenchyma cells. The achenes are approximately 1 mm in length, and each receptacle may contain a few hundred of them. The mature achene contains a hard and relatively thick pericarp, a thin testa, a single-layer endosperm, and a small embryo. Embryo formation is completed 10 d after anthesis, and it has been reported to store protein and fat but no starch (Nitsch, 1950). Receptacle growth follows either a single or a double sigmoid curve, depending on the cultivar. It is mainly the result of cell enlargement; however, cell division accounts for 15% to 20% of the total growth (Havis, 1943; Cheng and Breen, 1992).
During the early stages of strawberry fruit development, auxin, synthesized in the achenes, promotes fruit growth (Nitsch, 1950; Given et al., 1988). Free auxin levels peak in the receptacle and achenes prior to the white stage and subsequently decline as the fruit matures. It appears likely that the coordinated action between achene and receptacle is part of the mechanism ensuring achene maturation prior to fruit ripening. In support of this theory, it has been clearly demonstrated that declining auxin levels trigger ripening by inducing the de novo synthesis of several ripening-associated mRNAs (Manning, 1994, 1998; Aharoni et al., 2002a; Benítez-Burraco et al., 2003; Castillejo et al., 2004). The developmental changes during strawberry maturation are accompanied by massive and coordinated changes in the levels of transcripts related to primary and secondary metabolism (Aharoni and O'Connell, 2002).
In the case of primary metabolism, the major soluble sugars in strawberry are Glc, Fru, and Suc, and their content is significantly enhanced during the developmental processes (Hancock, 1999). In contrast, the levels of minor soluble sugars, such as inositol, Xyl, and Gal, decrease during maturation (Moing et al., 2001). The three main organic acids in the fruit are citrate (CitA), malate (MalA), and quinate (QuinA), while minor organic acids, including acetate, oxalate, succinate (SuccA), isocitrate, fumarate (FumA), and aconitate (AcoA), are also present at considerable levels (Moing et al., 2001). The content of sugars and organic acids and the ratios between them play a significant role in the overall flavor of fruit. Indeed, sugar content has previously been regarded as the major quantitative factor determining this parameter (Park et al., 2006). Furthermore, recent important advances have been achieved in understanding the impact of the metabolism of sugars (Park et al., 2006) and ascorbate (Valpuesta and Botella, 2004) in determining fruit quality. Amino acids are other soluble components that contribute significantly to fruit flavor. In the case of secondary metabolism, phenolic compounds provide the fruit with color, flavor, and protection against pathogenic attack and harsh environmental conditions such as UV light exposure (Aaby et al., 2005, 2007a). Phenolic compounds display the most noticeable metabolic changes during strawberry fruit development (Aaby et al., 2005, 2007a). During early stages, flavonoids, mainly condensed tannins, accumulate to high levels and provide immature fruit an astringent flavor (Cheng and Breen, 1991; Aharoni et al., 2002b; Almeida et al., 2007). Later in development, when fruit start to ripen, other flavonoids, such as anthocyanins and cinnamic and coumaric acid derivatives, and flavonols accumulate to high levels (Lunkenbein et al., 2006a; Almeida et al., 2007; Landmann et al., 2007). In strawberry, the biosynthesis of hundreds of flavor and aroma compounds is a major ripening-associated metabolic process and has been the subject of extensive research both at the chemical and molecular levels (Perez et al., 1999; Wein et al., 2002; Aharoni et al., 2004; Lunkenbein et al., 2006a, 2006b, 2006c). Nonetheless, a comprehensive view of the changes in the strawberry metabolic network across development is lacking. Furthermore, in spite of its vital role in the regulation of strawberry fruit development and ripening, metabolism in the achenes is, as yet, essentially unexplored (Aharoni and O'Connell, 2002). Only very recent studies have demonstrated that achenes contribute significantly to the polyphenol content and stability of strawberry purees (Aaby et al., 2007b). In recent years, the phytochemical resveratrol has become a focus of intense research, owing to its dual roles in promoting mammalian longevity and in cancer prevention (Gatz and Wiesmüller, 2008). Intriguingly, this compound was recently described to be present in achenes of several strawberry cultivars (Wang et al., 2007).
Metabolomics approaches, including those utilizing gas chromatography-mass spectrometry (GC-MS), liquid chromatography-mass spectrometry, NMR, and Fourier transform ion cyclotron resonance-mass spectrometry, now allow the profiling of numerous metabolites within a single experiment (recently reviewed by Bedair and Sumner, 2008). In this work, we carried out, to our knowledge, the first parallel profiling of primary and secondary metabolites simultaneously in receptacle and achene extracts across strawberry development. To achieve this, we optimized a preestablished GC-MS protocol as well as an ultraperformance liquid chromatography coupled with Q-TOF (time of flight) mass spectrometry (UPLC-QTOF-MS)-based method. During early stages of development, the level of coordinated control across metabolism was surprising, with almost all pathways appearing to be tightly regulated. This held true for both primary and secondary metabolites and even at the level of occurrence of side chain substitutions within the secondary metabolites. That said, the level of coordination within distinct metabolic pathways clearly declined in later phases. The biological significance of these changes is discussed within the context of current models of nonclimacteric fruit ripening.
RESULTS
GC-MS and UPLC-QTOF-MS Analyses of Strawberry Achene and Receptacle Reveals Primary and Secondary Metabolic Profiles That Are Characteristic to Organ and Developmental Stage
To follow the repertoire of metabolic changes that occur in parallel in strawberry achene and receptacle organs, we carried out extensive metabolic profiling in which both primary and secondary metabolism were investigated during fruit development. Strawberry fruit growth and maturation can be divided into six different stages: small green (SG), medium green (MG), big green (BG), white (Wh), turning (Tu), and red (Re; Fig. 1). Under our growth conditions, the development of fruit from anthesis to the red ripe stage encompassed an average period of 30 d. The ripening process in strawberry is relatively rapid and typically occurs 5 to 10 d following the white stage. Fruit at any of the six stages of development were harvested five times during the season (i.e. harvested in a period of 4 months) and were separated to achenes and receptacle prior to extraction and metabolite determination (Fig. 1). GC-MS and UPLC-QTOF-MS analyses were conducted to detect mainly primary and secondary metabolites, respectively.
Figure 1.
Strawberry development. Plants of the Israeli strawberry cultivar Herut were grown in a plastic tunnel in the Sharon coastal area in Israel. Following fruit harvest at the different developmental stages, achenes and receptacle were separated and processed in parallel for GC-MS and UPLC-QTOF-MS analyses. The developmental stages shown here are, from left to right, SG, MG, BG, Wh, Tu, and Re.
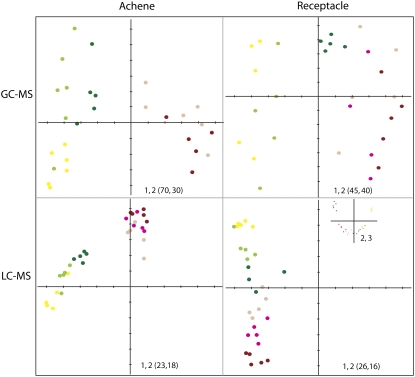
The data sets obtained by GC-MS and UPLC-QTOF-MS were examined by principal component analysis (Fig. 2). In both types of analyses, samples derived from the various stages of receptacle and achene development were clearly separated on the basis of metabolic differences. The analysis further highlighted a clear metabolic shift, in both the UPLC-QTOF-MS and GC-MS data, between the first three stages (SG, MG, BG) and the later stages (Wh, Tu, Re) in either organ. The metabolic events occurring in the achene and receptacle are best exemplified by the metabolites (primary or secondary) with highest principal component analysis scores and ANOVA P values in each organ (i.e. those metabolites with main impact on the variance of the data set; Supplemental Table S1). In the receptacle data set, sugars and sugar phosphates, ascorbate (AscA), and the shikimate precursor QuinA accounted for the main changes in primary metabolism. On the contrary, in the achene data set, amino acids such as Glu, Asp, and Asn and tricarboxylic acid (TCA) cycle intermediates had the highest impact on the metabolic shift. Furthermore, the number of primary metabolites that significantly changed during achene development (P < 0.01, following Bonferroni correction) was 1.5-fold higher than that detected in the receptacle (Supplemental Table S1).
Figure 2.
Principal component analysis of GC-MS and UPLC-QTOF-MS data. Strawberry achenes and receptacle were collected at different developmental time points. Yellow, light green, dark green, pink, fuschia, and bordeaux dots represent SG, MG, BG, Wh, Tu, and Re, respectively. The variance explained by each component is given within parentheses.
Changes in secondary metabolism were also organ specific (Supplemental Table S2). However, as opposed to the results obtained for primary metabolism, larger changes in the levels of secondary metabolites were found in the receptacle compared with the achenes. In the receptacle, the secondary metabolites that exhibited highest variation in content across development included anthocyanins, procyanidins, phenolic acids, and two terpenoid derivatives. In the achenes, the secondary metabolites that exhibited highest fluctuations included catechin, phenylpropanoid glucosides, kaempferol coumaroyl glucoside, and several ellagitannins.
A Major Difference Exists between the Activity of Primary Metabolism in the Receptacle and Achenes
When comparing the metabolic shifts occurring throughout the development of receptacle and achenes, characteristic changes in patterns of metabolite accumulation were revealed to be common between the two different organs. However, in certain cases, such as sugar metabolism, the trends of metabolic change were even opposite. In the following sections, the changes in metabolite composition during the maturation of both organs are compared.
Metabolism of Sugar, Sugar Alcohols, and Phosphate Moieties
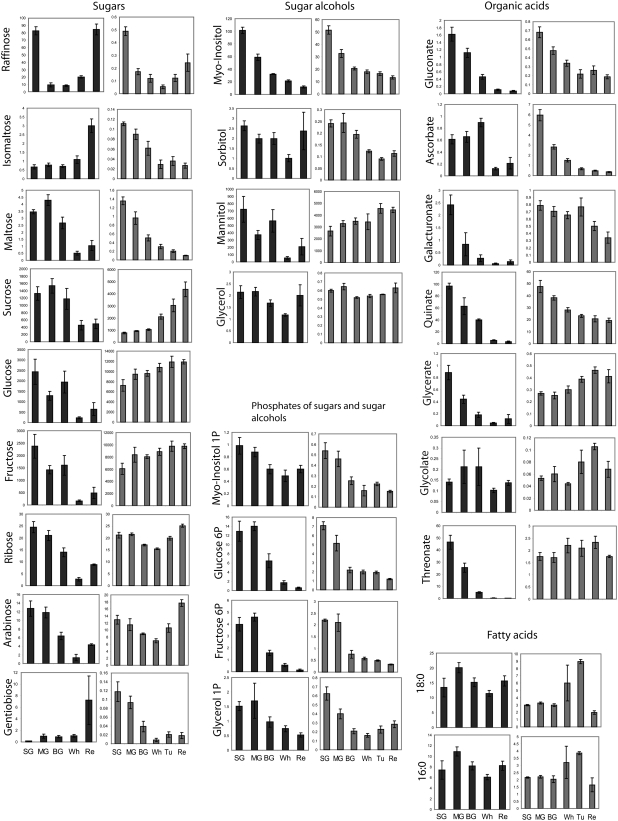
In the receptacle, sugars were the metabolites that exhibited the highest degree of variance during development (Supplemental Table S1). While sugars such as isomaltose (iMal), maltose (Mal), and gentiobiose exhibited a steady but dramatic decrease during receptacle development, Suc, Glc, and Fru (which comprise the major soluble sugars in strawberry) increased (Fig. 3). This accumulation is also characteristic of the development of climacteric fruit such as tomato (Solanum lycopersicum; Carrari et al., 2006; Carrari and Fernie, 2006). The content of raffinose (Raf), Rib, and Ara initially decreased through the SG to BG stages but increased thereafter (Fig. 3). The levels of sugar phosphates and sugar alcohols were significantly reduced during receptacle development. This trend was countered by a steady accumulation of fatty acids peaking at the Tu stage, suggesting an important role for glycolysis in fatty acid biosynthesis in this organ.
Figure 3.
Primary metabolism during strawberry development. Changes in relative contents of primary metabolites in achenes (black bars) and receptacle (gray bars) during strawberry development detected by GC-MS. Relative content is given as the metabolite response (see “Materials and Methods”). The bars for each organ type represent metabolite contents across the following stages of development (left to right): SG, MG, BG, Wh, Tu, and Re.
The accumulation patterns of the various sugars measured during achene development were diverse and in most cases different from those observed in the receptacle. Suc and hexose phosphates were measured to decrease by 50% their initial levels at the BG stage. Their level was further reduced during late achene maturation (Fig. 3). In contrast, a few important sugars and storage compounds, including Raf, iMal, sorbitol, glycerol, and the oligosaccharine gentiobiose, reached significantly higher abundance in the mature achene.
Accumulation Profiles of Proteinogenic and Nonproteinogenic Amino Acids and Polyamines
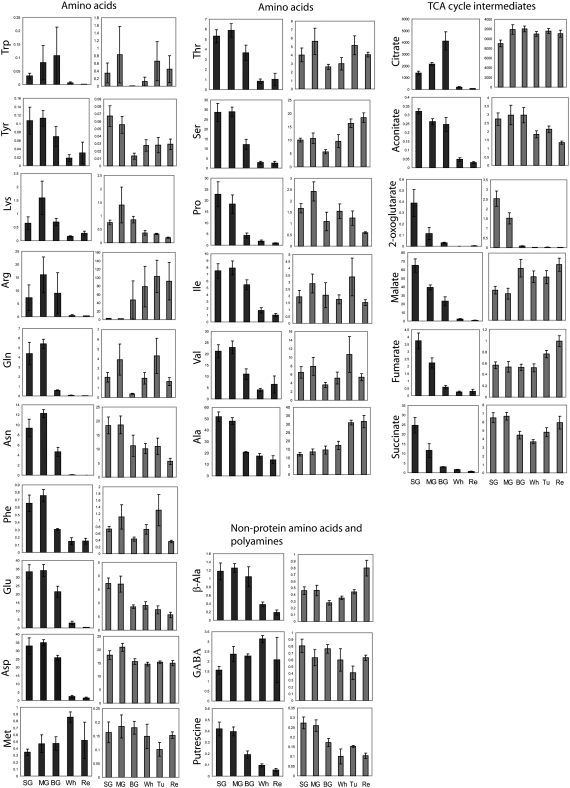
The 16 amino acids we were able to measure by GC-MS revealed marked alterations in levels in both the receptacle and the achenes during development and ripening (Fig. 3). In the receptacle, three main patterns of amino acid accumulation were apparent: (1) those decreasing throughout development, (2) those increasing during ripening, and (3) those exhibiting a double sigmoid trend of accumulation, peaking first at the MG stage and later at the Tu stage. Group 1 was dominated by Glu, Asn, and Tyr. Group 2 consisted of Arg, Ser, and Ala. Group 3 was characterized by a clear double sigmoid behavior of Gln, the shikimate-related aromatic amino acids Trp and Phe (the latter reported previously to exhibit a double sigmoid trend [Aharoni et al., 2002b]), in addition to Thr, Val, Ile, and Pro. Amino acids characteristic of the Asp family pathway (i.e. Asp, Lys, and Met) accumulated early in development, subsequently decreasing until the Re stage. The polyamine putrescine and β-Ala belonged to group 1 and group 2, respectively, while the closely related nonprotein amino acid 4-aminobutyrate (GABA) decreased gradually throughout maturation.
In the achenes, the pattern of amino acid accumulation was fairly similar to that described for the receptacle. The most typical pattern of change was an increase in their levels toward the MG stage of development and a sharp decline when ripening commenced in the BG stage (Fig. 3). Such a characteristic pattern was particularly evident for the N-rich amino acids Lys, Arg, Asn, and Gln (Fig. 3). Among the amino-containing compounds, GABA exhibited an exceptional pattern, accumulating to 2-fold its initial content in the Re stage of achene development.
Accumulation Profiles of Organic Acids and TCA Cycle Intermediates
In contrast to climacteric tomato fruit (Carrari et al., 2006), the levels of the TCA cycle intermediates in strawberry exhibited relatively small changes during the development of the receptacle (Fig. 3). Maintained at relatively high abundance in early stages, FumA, and MalA increased during maturation, whereas AcoA levels dropped slightly in later stages. SuccA content displayed an inverse bell curve pattern of change, while CitA, the main organic acid in strawberry, remained at steady levels throughout development. These changes were contrasted by a 10-fold and more drop of 2-oxoglutarate starting as early as the BG stage. Additional organic acids that significantly decreased during receptacle development were AscA, gluconate, galacturonate, and the shikimate precursor QuinA (Fig. 3).
In the achene, a general reduction in the levels of organic acids, predominantly TCA cycle intermediates, was evident during fruit maturation (Fig. 3). In contrast to the trend displayed by this class of metabolites within the receptacle, a pronounced decrease in content characterized TCA cycle intermediates from the very early stages under study (SG), with the exception of CitA. For example, we observed a 10-fold decrease in AcoA, SuccA, and FumA and a 60-fold decrease in MalA. In contrast, CitA accumulated until mid maturation (MG stage; 4-fold higher compared with its levels in the SM stage) and exhibited a 40-fold drop in its levels in the following stages. This pattern was also evident for AscA. Early reports suggested that CitA acts as a competitive inhibitor of AscA oxidase (Gerwin et al., 1974); thus, increasing CitA content could conceivably reduce the rate of AscA oxidation and thereby lead to AscA accumulation. The decrease in many organic acids during maturation in the achenes was found to be coupled to an early accumulation of fatty acids 16:0 and 18:0, which was followed by a subsequent reduction in their content during mid to late maturation. This is an opposite trend to the pattern of changes observed in the receptacle and could possibly occur to support oil biosynthesis at this stage. Eventually, fatty acids accumulated in the mature achenes (Re stage; Fig. 3).
Parallel Accumulation of Secondary Metabolites in the Developing Strawberry Achenes and Receptacle
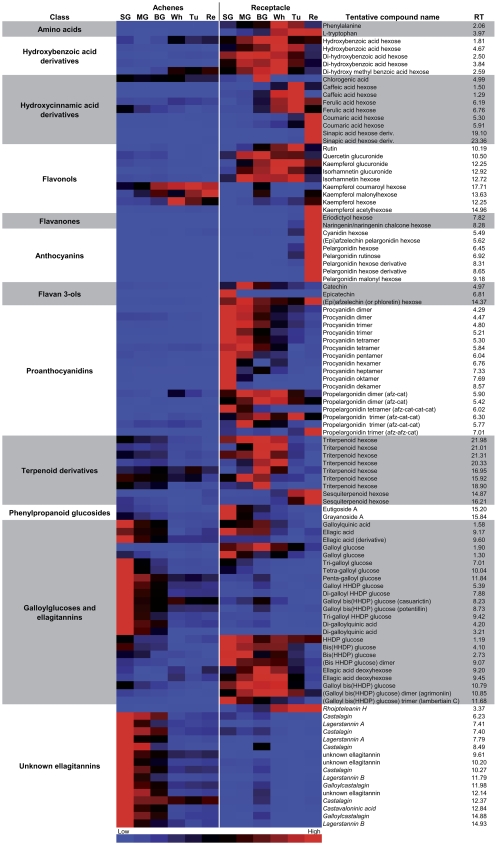
Strawberry fruit is a rich source of secondary metabolites, a large number of those identified to date belonging to the phenolic class (Aharoni et al., 2002b; Aaby et al., 2007a). The parallel analysis of achene and receptacle extracts using UPLC-QTOF-MS enabled the detection of a large number of different phenolic compounds, mainly derivatives of the phenylpropanoid pathway and ellagitannin biosynthesis. Altogether, 105 peaks were assigned putative identifications, all of which showed differential distribution during development of either organ (for detailed information regarding these metabolites, see Supplemental Table S3). The heat map presented in Figure 4 clearly shows that the receptacle is a richer source of these phenolic compounds than the achene. The accumulation profiles of selected representatives of each metabolite class are shown in Figure 5. While a number of secondary metabolites were almost exclusively detected in the receptacle (e.g. coumaric acid hexose, sinapic acid hexose derivative, naringenin hexose, pelargonidin hexose and rutinose, and propelargonidin tetramer), a few others, including the flavonol kaempferol coumaroyl hexose, di-galloylquinic acid, and several unknown ellagitannins, were highly enriched in the achenes (Fig. 4). Due to the semiquantitative nature of the nontargeted UPLC-QTOF-MS analysis, the levels of metabolites were compared between the different organs but not among metabolite classes.
Figure 4.
Distribution of tentatively identified secondary metabolites in strawberry achenes and receptacle at different developmental stages. The colors indicate the proportional content of each putatively identified metabolite among the samples as determined by the average peak response area during UPLC-QTOF-MS. The lowest response area receives light blue, and the highest response area receives light red (see color bar at bottom). The exact values for each metabolite peak area are provided in Supplemental Table S3. The compound names in italics represent single matches in the Dictionary of Natural Products database that were not verified by MS/MS analysis.
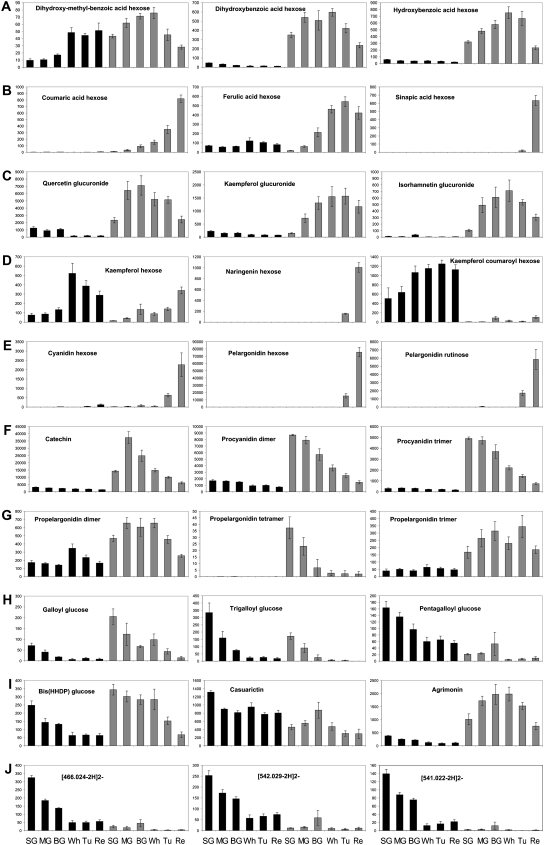
Figure 5.
Representative secondary metabolites detected by UPLC-QTOF-MS. Dihydroxybenzoic acid derivatives (A), hydroxycinnamic acid derivatives (B), flavonol glucuronide derivatives (C), other flavonoid pathway compounds (D), anthocyanidin derivatives (E), procyanidins (F), propelagonidins (G), galloyl Glcs (H), identified ellagitannins (I), and unidentified ellagitannins (J). Amounts are presented as relative peak response areas of the molecular ion of each compound. Black bars represent achenes, and gray bars represent receptacle. The bars for each organ type represent the stage of development from left to right in the following order: SG, MG, BG, Wh, Tu, and Re.
Phenolic Acids and Aromatic Amino Acids
The strawberry receptacle accumulates a range of glycosylated hydroxybenzoic and hydroxycinnamic acid derivatives (Fig. 4). The hydroxybenzoic acid derivatives were more abundant at the early to mid stages of receptacle development (SG to Wh), while the hexose sugar-containing caffeic and ferulic acids accumulated at the Wh and Tu maturation stages. The coumaric acid and sinapic acid derivatives were detected almost exclusively in the ripe stage of the receptacle (Figs. 4 and 5A). Chlorogenic acid, a different hydroxycinnamic acid derivative, showed a unique profile and accumulated specifically in the BG stage in the receptacle. Most of the phenolics detected here are derived from the aromatic amino acid Phe, which exhibited a double sigmoid curve of accumulation during receptacle development (Fig. 3). Its levels in the receptacle decreased very dramatically between the SG and MG and the Tu and Re stages. Several of the metabolites described above also accumulated in the achenes, albeit to lower levels. For example, chlorogenic acid was detected in all stages of achene development, while dihydroxy methyl benzoic acid hexose accumulated in the three latter stages of achene development. By contrast, ferulic acid hexose was detected particularly in the early, SG, stage of achene development (Fig. 4).
Flavonoids
The flavonoids are the most widely represented class of secondary metabolites in the strawberry receptacle. Derivatives of the flavonols kaempferol, quercetin, and isorhamnetin were present in both receptacle and achenes at all stages with varying substitutions. Several derivatives were detected solely in the receptacle (particularly from the MG stage to the Tu stage), for example, the glucuronide derivatives of all three flavonols (Fig. 5C). In contrast, three of the kaempferol derivatives (hexose, malonylhexose, and coumaroyl-Glc) were detected predominantly in the achenes, mainly in late stages of development (Figs. 4 and 5D). The flavonol precursors, eriodictyol hexose and naringenin/naringenin chalcone hexose, as well as kaempferol acetylhexose were detected only at the mature Re stage of the receptacle (Fig. 4).
Pigmentation of the receptacle during ripening in most strawberry cultivars is mainly due to the accumulation of glycosylated pelargonidins and a small fraction of glycosylated cyanidins, which, in our study, were both detected at the mature fruit. In addition to the hexose-sugar derivative of pelargonidin, five other differentially derivatized forms of pelargonidin were detected, while cyanidin was present solely as hexose substitutes. A relatively less studied anthocyanin derivative reported earlier in strawberry (Fossen et al., 2004) is the proanthocyanidin-anthocyanin conjugate. Such a metabolite, (epi)afzelechin pelargonidin hexose, was tentatively identified in the Re-stage receptacle (Fig. 4; Supplemental Table S3). In the achenes, pigments such as cyanidin hexose accumulated during maturation, although in remarkably smaller amounts compared with the receptacle (Figs. 1, 4, and 5E).
Proanthocyanidins, end products of the flavonoid pathway, were detected in our analysis as polymers, containing up to 10 units. Procyanidins accumulated mainly in the early SG and MG stages of receptacle development (Figs. 4 and 5F). In addition to procyanidin homopolymers, heteropolymers containing at least one (epi)afzelechin subunit (propelargonidins) have also been reported in strawberry fruit (Gu et al., 2003). In our study, the accumulation of propelargonidins in the receptacle differed from that of procyanidins, being detected at relatively low levels, mainly during late maturation (Figs. 4 and 5G).
Ellagitannins
Ellagitannin derivatives exhibited unusual patterns of accumulation during development of the two strawberry fruit organs. While several simple ellagitannins, including hexahydroxydipenoyl (HHDP) Glc, bis (HHDP) Glc, and its dimerized form, were mainly detected in the receptacle, galloyl HHDP Glc, di-galloyl HHDP Glc, and tri-galloyl HHDP Glc accumulated in the achenes (Figs. 4 and 5). The ellagitannin derivatives were strongly enriched in the early SG stage of achene development, while those in the receptacle accumulated starting from the SG stage, in some cases even to high levels in the Tu and Re stages. We also observed an unusual accumulation of the ellagitannin precursors, galloyl Glcs. While the single galloylated form was mainly produced in the receptacle, the form with the highest galloylation degree (penta-galloyl Glc) was mostly produced by the achenes (Figs. 4 and 5H). Some ellagitannins previously reported in strawberry fruit, such as casuarictin and potentillin, were predominantly detected in the achene extract (Fig. 5I; Supplemental Table S3). Moreover, we detected many peaks that possessed the typical ellagitannin profile. These were not characterized previously from strawberry and were highly abundant in the early stages of achene development (Figs. 4 and 5J).
Classes of Secondary Metabolites Previously Nondetected in Strawberry Fruit
Apart from the various classes of secondary metabolites that were detected previously in strawberry (Aharoni et al., 2002b; Määttä-Riihinen et al., 2004; Aaby et al., 2007a; Hukkanen et al., 2007), we also detected metabolites in strawberry receptacle and achenes that belong to chemical classes not described before in strawberry fruit. Glucosylated triterpenoids (saponins) were detected in the middle phase of receptacle development (MG to Wh stage), and their levels declined dramatically in the Tu stage (Fig. 4). Two glycosylated sesquiterpenoids, tentatively identified (Supplemental Table S3) as eudesmanetriol or farnesadiene derivatives (molecular weight 418; retention time 14.9 and 16.2), accumulated mainly in late stages of receptacle development (Tu and Re stages; Fig. 4).
Two phenylpropanoid glycosides (eutigoisde A and grayanoisde A) were also detected in both organs and were particularly abundant in the MG stage of receptacle development (Fig. 4). The identity of these metabolites was confirmed in a different study of strawberry leaves by the use of NMR analysis (K. Hanhineva and A. Aharoni, unpublished data).
Correlation and Cluster Analyses of Combined Primary and Secondary Metabolite Data Sets
Correlation analysis was performed on the entire data set collected for the two organs. Association of metabolites across development is reflected in the degree of similarity in accumulation patterns. The correlation analysis was carried out by calculating the Pearson correlation coefficient for each and every metabolite pair (Carrari et al., 2006).
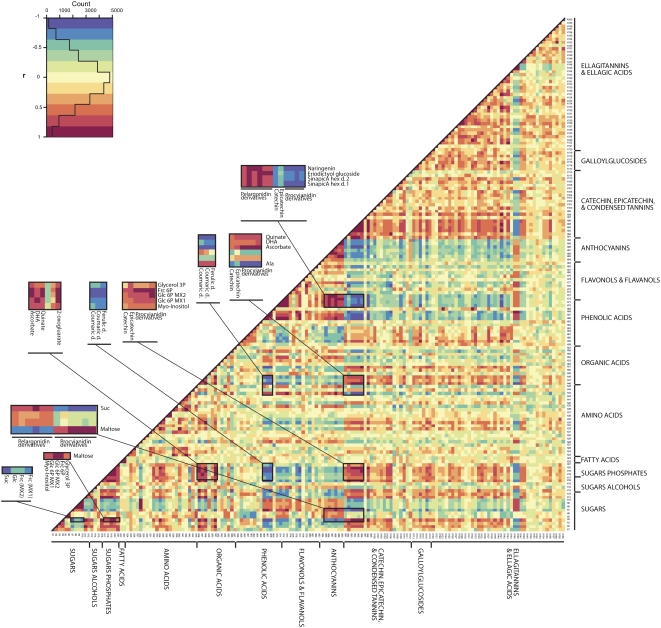
In the receptacle data set, significant metabolite-metabolite correlations were less frequent and less pronounced compared with the achene data set (Fig. 6 versus Fig. 7; Supplemental Table S4 versus Supplemental Table S5). That said, the levels of certain metabolites, including hexose phosphate, Suc, and Mal, displayed a reasonable number of correlations with metabolites across both primary and secondary metabolism (Fig. 6, enlarged sections). However, relatively few correlations were apparent for amino acids, and those observed were only medium in strength (correlation coefficients between 0.8 and 0.9; Fig. 6; Supplemental Table S4).
Figure 6.
Visualization of metabolite-metabolite correlations during receptacle development. Heat map of metabolite-metabolite correlations along the developmental period of strawberry fruit. Metabolites were grouped by compound class, and each square represents the correlation between the metabolite heading the column and the metabolite heading the row. Correlation coefficients were calculated by applying the Pearson algorithm using R software. Each square indicates a given r value resulting from a Pearson correlation analysis in a false color scale (see color key at top left). The identity of each metabolite and the correlation coefficient score are given in Supplemental Table S5. Amino acids are listed in standard three-letter abbreviations. BenzA, Benzoic acid; d., derivative; DHA, dehydroascorbate; Frc, Fru; hex, hexose; OH, hydroxy; P, phosphate; Put, putrescine.
Figure 7.
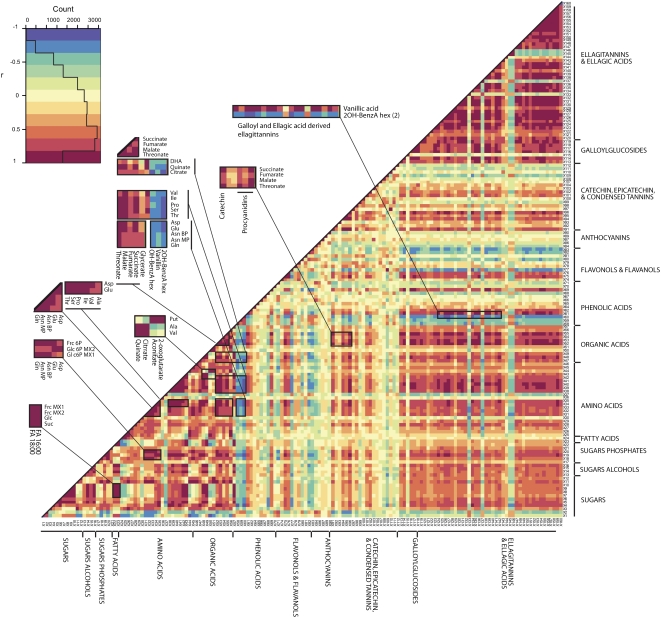
Visualization of metabolite-metabolite correlations during achene development. Heat map of metabolite-metabolite correlations along the developmental period of strawberry fruit. Metabolites were grouped by compound class, and each square represents the correlation between the metabolite heading the column and the metabolite heading the row. Correlation coefficients were calculated by applying the Pearson algorithm using R software. Each square indicates a given r value resulting from a Pearson correlation analysis in a false color scale (see color key at top left). The identity of each metabolite and the correlation coefficient score are given in Supplemental Table S4. Amino acids are listed in standard three-letter abbreviations. For other abbreviations, see Figure 6 legend.
During achene development, we found high correlations within the amino acids category (e.g. Asn:Asp:Gln; Fig. 7; Supplemental Table S5). Similarly, organic acids were associated significantly within their category (e.g. FumA∷SuccA:MalA) but also displayed extensive similarity in the pattern of change with several amino acids (e.g. enlarged sections MalA:Ile, MalA:Ser, CitA:Val, CitA:Ala; Fig. 7; Supplemental Table S5). As might be expected, the hexose phosphates (X18 to X20 in the heat map) displayed significant correlation with other sugars and sugar derivatives, such as threonate (ThrA; X52 in the heat map) and myoinositol (Myo-Ino; X16 in the heat map), but also with a large number of metabolites across different categories, including amino acids (see enlarged sections). This suggests a coregulation of the glycolytic events and other metabolic processes such as amino acid metabolism during achene development. Metabolite-metabolite associations were positive in the vast majority of cases with the exception of those involving Raf (X1 in the heat map), a storage-related compound, which displayed significant negative correlation with hexose phosphates, amino acids, and organic acids (Fig. 7; Supplemental Table S5). In contrast to primary metabolism, among the secondary metabolite categories, negative correlations were detected between a first group composed of phenolic acids (coumaric, ferulic, and sinapic acid derivatives), eriodictyol hexose, naringenin/naringenin chalcone hexose, kaempferol hexose, and pelargonidin hexose derivative and a second group characterized by procyanidin derivatives (Fig. 7; Supplemental Table S5).
The correlation analysis also highlighted links between primary and secondary metabolism, and significant metabolite-metabolite correlations were found in both receptacles and achenes. In the receptacle, significant correlations were found among metabolites across different metabolic categories in the primary metabolism (including sugars, hexose phosphates, organic acids [e.g. QuinA, CitA, and 2-oxoglutarate], and the amino acid Ala) and the secondary metabolites coumaric and ferulic acid hexoses, kaempferol hexoses, and procyanidin derivatives (Fig. 6; Supplemental Table S5). With respect to the nature of the association between primary and secondary metabolites, correlations involving Ala were opposite to those involving, for example organic acids. Similarly, correlations involving Suc were found inverse to those involving other sugars or sugar phosphates (Supplemental Table S5).
In the achene, significant correlations were detected between several amino acids and secondary intermediates across different categories, for example, Glu (X34), Asp (X35) Thr (X38), Ser (X39), Pro (X40), and Ile (X41) and quercetin glucuronide (X75), phenolic derivatives (Fig. 7, enlarged section), digalloylquinic acid (X116), galloylglucosides, and ellagitannins (Fig. 7; Supplemental Table S5). The pattern of change of these secondary intermediates was shown to follow that of organic acids like QuinA, ThrA, MalA, and SuccA and Glc-6-P. Dihydroxy methyl benzoic acid hexose was exceptionally negatively correlated with the above-mentioned primary intermediates (Fig. 7, enlarged section).
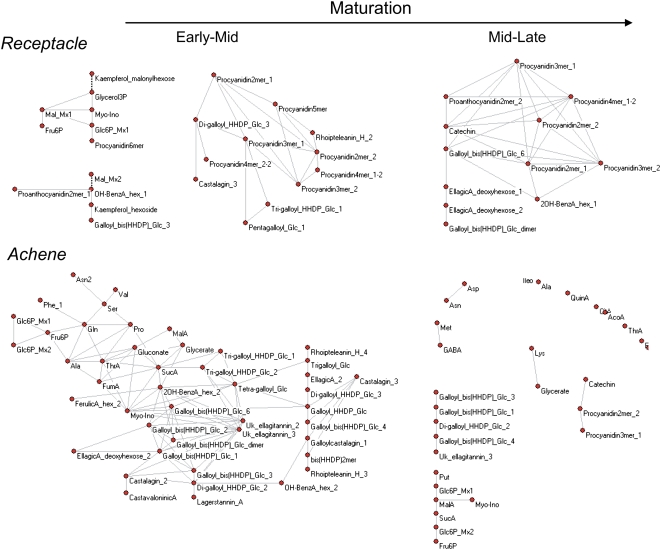
In order to evaluate these data further, we examined the metabolite correlation networks in early and late stages of development in both organs. Such networks can provide insight into biochemical processes and their regulation (Camacho, 2005; Steuer, 2006) and, therefore, were implemented in an effort to further explore the significance of metabolite-metabolite associations. For this purpose, we used a connectivity threshold fixed at the absolute level of Pearson correlation coefficient |R| > 0.9 (a parameter that is particularly important in this instance due to the relatively limited sample size) and assembled networks for both early to mid (SG to MG) and mid to late (MG to Re) maturation stages in each organ. The resultant matrices (Fig. 8) revealed a dense early development correlation network, with 50 and 117 significant connections in receptacle and achenes, respectively. During mid to late maturation, connections were reduced to 49 and 28 in receptacle and achenes, respectively. Detailed evaluation of these networks reveals the presence of small modules, including, in the receptacle, glycolysis and proanthocyanin metabolism, and in the achenes, glycolysis, amino and organic acids, and ellagitannin precursors. Furthermore, a secondary metabolism module in the receptacle, represented by the proanthocyanin, was maintained throughout development, as was the primary metabolism module, including glycolysis and TCA cycle components, in the achenes.
Figure 8.
Network visualization of pair-wise correlations between metabolites in receptacle and achenes of strawberry fruit during early to mid and mid to late development. In the network reconstruction procedure, two metabolites are connected when the Pearson correlation coefficient is |R| > 0.9. Solid and dashed lines indicate positive and negative correlations, respectively. The degree of connectivity for each metabolite is given in Supplemental Table S6. [See online article for color version of this figure.]
Thus, this analysis agrees with the point-by-point description above in suggesting that metabolism in the receptacle is not highly coordinated either in early or late stages of development but that there is a high degree of coordination of the metabolic shifts occurring during early achene development (albeit one that breaks down at later stages of development).
DISCUSSION
The interrelation between ovary growth that is followed by fruit maturation and the parallel interdependent development of ovules and seeds is a highly complex process. Although the strawberry receptacle is not a “real” fruit and the achenes cannot strictly be defined as “real” seeds, this work along with previous extensive gene expression studies suggest that they could serve as an excellent model for studying the regulation of synchronous developmental processes in fruit and seeds (Aharoni and O'Connell, 2002). Phytohormones are good candidates to play a major role in the metabolic interplay between developing fruit and seeds, as exemplified in the classical experiments of Nitsch (1950). Removal of achenes or application of auxin to areas from which achenes were manually removed at various stages of fruit development could accelerate or repress ovary growth and ripening. Regardless of hormonal signaling, other signaling molecules are likely to play a role in the fruit-seed cross talk, including mRNAs, several types of small and noncoding RNAs, peptides, and proteins (Lindsey, 2001; Jones-Rhoades et al., 2006; Corbesier et al., 2007; Tamaki et al., 2007; Kalantidis et al., 2008). The spatial and temporal production and accumulation of small molecules (other than hormones) is an additional key factor that could mediate the regulation of the concerted development of fruit and seeds, with much recent work in other species highlighting the importance of sugar and amino acid transporters linking seeds to the nutrient supply of the mother plant (Borisjuk et al., 2004; Gibson, 2005; Weber et al., 2005). Here, we carried out extensive metabolic profiling experiments across defined developmental stages in dissected achenes and receptacle. Our aim was to characterize the developmental reconfigurations of the metabolic network in the two organs.
Metabolism in the Receptacle and Achenes during the Early Stages of Fruit Maturation
The developing receptacle exhibits considerable changes in the metabolism of sugars and sugar phosphates. The major soluble sugars in strawberry are Glc, Fru, and Suc (Hancock, 1999; Macías-Rodríguez et al., 2002), whose mobilization rate supports (or limits) fruit development and ripening (Forney and Breen, 1985, 1986). Consistent with earlier studies (Hancock, 2000), the results we present here revealed a gradual accumulation of these sugars coupled to a dramatic decrease in the levels of associated sugar phosphates. The levels of phosphate moieties have been documented previously to be dramatically reduced also during tomato fruit development (Roessner-Tunali et al., 2003; Carrari et al., 2006). For instance, Myo-Ino and its phosphorylated derivatives are of special interest. Myo-Ino is involved in various cellular processes, among others, signal transduction and auxin metabolism (Gomez-Merino et al., 2005). Myo-Ino 1-phosphate is one of the very few metabolites (assessed by GC-MS) that was associated with yield parameters in a quantitative trait loci study of tomato introgression lines (Schauer et al., 2006). The major decreases of 80% and 40% in Myo-Ino and its phosphate form, respectively, in early to mid maturation of strawberry receptacle detected here point to the involvement of these components in developmental changes of strawberry fruit prior to the ripening stage. On the other hand, the steady decrease observed in AscA and the relatively minor changes in dehydroascorbate (its oxidized form) and ThrA (a product of AscA) throughout development suggest high redox activity, possibly linked to cell expansion, fruit softening, and cell wall loosening of the receptacle tissues (Green and Fry, 2005). The increase in Ara at late maturation could further support cell wall loosening, given the link between Ara level and cell wall loosening (Knee et al., 1977). Alternatively, a possible recycling of AscA-derived carbon via oxalate or ThrA and the hexose pool is possible (Debolt et al., 2007), as is suggested by the high connectivity of ThrA within the C/N cluster in the connectivity network presented in Figure 8.
A characteristic feature of the early stages of receptacle development is the abundance of the flavonoid pathway end products, particularly the proanthocyanidins. The occurrence of proanthocyanidins in the early developmental stages of the fruit is common to several plant species (Dixon et al., 2005; Almeida et al., 2007), possibly protecting the developing fruit from frugivores.
The appearance, in the receptacle, of intermediates of the phenylpropanoid and flavonoid pathways in this study is compatible with previous gene expression studies (Halbwirth et al., 2006; Almeida et al., 2007). In addition to these well-characterized secondary metabolites, we were able to detect the presence of molecules that were tentatively identified as triterpenoid hexoses as well as the phenylpropanoid glucosides eutigoside A and grayanoside A. The latter two compounds have an unknown function in early maturation in strawberry fruit, since studies of their in vivo function are very limited. Triterpenoids are generally thought to play an important role in the plant defense against bacterial and fungal pathogens (Osbourn, 2003; Sparg et al., 2004).
In view of its role as a dispersal unit, primary metabolism in the achene might follow a program dedicated to the accumulation of storage compounds. During the early stages of achene development (SG–BG), we measured an increase in the content of amino acids such as Arg, Asn, and Gln, possibly supported by the import of nitrogen-rich compounds from the mother plant. This process could be mediated by the high abundance of ATP-binding cassette transporters in the achene (Aharoni and O'Connell, 2002). A similar pattern of change in content was measured for CitA and might reflect a rapid increase in the demand of carbon to support ammonia assimilation at this time point (Noctor et al., 2007b). CitA is likely to accumulate as a product of high rates of glycolysis and to support fatty acid production. Furthermore, the dramatic decrease of SuccA, FumA, and 2-oxoglutarate also is suggestive of a heavy demand for the carbon skeleton.
The similarities with the other seed systems are reflected also in the pattern of change of sugars and sugar phosphates. Indeed, Suc and Mal were shown to transiently accumulate in the early maturation events, while Fru and Glc were converted to their phosphate forms. This metabolic pattern is probably best explained by an import of sugar moieties into the young achene and the limited operation of a not yet fully active glycolytic pathway in this organ, as has been described in legumes (Borisjuk et al., 2004) and suggested in Arabidopsis (Fait et al., 2006). The subsequent reduction in hexose phosphates, coupled with a decrease in Suc and a steady state of the intermediates, Fru and Glc, are suggestive of an increase in the metabolic/redox activity of the achene to provide (via CitA and amino acids) the building blocks for storage proteins and lipids. Our metabolic analysis supports and integrates with the genetic programs unveiled during achene development in a previous transcriptome analysis (Aharoni and O'Connell, 2002), namely transport, storage, and carbohydrate metabolism.
During early stages of achene development, there was little alteration in the levels of secondary metabolites, with the exception of metabolites belonging to the ellagitannin group of hydrolyzable tannins (Figs. 4 and 5, H–J) and dihydroxybenzoic acid hexose as well as the flavonoid kaempferol derivatives, which showed mild fluctuations. The sugar-containing ellagitannins were found to be particularly abundant during early achene development, decreasing to 50% of their initial levels by mid maturation. In addition to this quantitative change, we observed qualitative difference in this class of metabolites, with, for example, penta-galloyl Glc being abundant during early achene development but not in the receptacle, suggesting divergent ellagitannin production that potentially functions in the two organs. While Rosaceae family plants including strawberry are a well-known source of ellagitannins (Haslam and Cai, 1994; Mullen et al., 2003; Hukkanen et al., 2007), to our knowledge, this study is the first to identify strawberry achene as a source of this class of metabolites.
Metabolism in the Receptacle and Achenes during Mid to Late Fruit Maturation
In the receptacle, the primary metabolism of mid to late maturation (MG to Re) was characterized by an increase of several amino acids (Arg, Ser, Thr, Ala, β-Ala, and, to some extent, GABA) as well as all TCA cycle intermediates with the exception of 2-oxoglutarate. Interestingly, apart from playing myriad roles in the fruit itself, CitA and MalA are important components of the organoleptic properties of the fruit, influencing its acidity. Given the relative changes in CitA and MalA across development observed here, we suggest that pH regulation is more likely driven by the latter, at least in the strawberry variety under study. Similarly, levels of Suc, Fru, Glc, Rib, and Raf, as well as the sugar alcohols such as mannitol and glycerol, increase at this stage, and they are also important in determining the taste of the mature fruit. As observed previously in early receptacle development, a decrease in AscA and the relatively minor changes in dehydroascorbate (its oxidized form) and ThrA (a product of AscA) throughout mid development suggest continued redox activity and carbon recycling.
Among the secondary metabolites detected during the mid and late stages of receptacle development, hexose derivatives of several different aromatic acids, including benzoic, hydroxybenzoic, caffeic, coumaric, ferulic, and sinapic acids, were observed to accumulate. These aromatic acids serve as precursors for the various branches of the phenylpropanoid pathway and the metabolism of phenolics. Elevated levels of caffeic, ferulic, and sinapic acid hexoses might contribute to monolignol biosynthesis and lignin formation in the fruit vasculature (Humphrey and Chapple, 2002; Gross, 2007). Aharoni et al. (2002a) provided evidence for the induced expression of genes putatively involved in lignification during ripening in both receptacle and achenes (Aharoni et al., 2002a; Blanco-Portales et al., 2002).
An increase in specific flavinoids and their derivatives characterized mid to late maturation of the strawberry receptacle. The biosynthesis of flavonoids is initiated by the activity of chalcone synthase, and the concomitant processing by specialized flavonoid pathway enzymes determines the end products (Dana et al., 2006). In strawberry, this category includes derivatives that result directly from naringenin (kaempferol, afzelechin, pelargonidin) or 3′ hydroxylated naringenin, eriodictyol (quercetin, catechin, cyanidin; Deng and Davis, 2001). Flavonoids contribute to multiple aspects of plant physiology, including protection of the plant against biotic and abiotic stress (Dixon and Paiva, 1995), plant growth (Besseau et al., 2007), flowering (Mo et al., 1992), and seed development (Lepiniec et al., 2006). All flavonol derivatives we detected in the receptacle were kaempferol, quercetin, and isorhamnetin derivatives. They occurred mainly as glucuronides and accumulated to the highest levels during mid maturation. Kaempferol hexose and acetylhexose were detected in mature receptacle along with anthocyanins and might be involved in the stabilization of anthocyanins in mature fruit. The early precursors of the flavonoid pathway, naringenin chalcone and eriodictyol, were observed almost solely at the ripe stage in the receptacle and could serve as precursors for the intensive production of anthocyanins. We also detected two putative sesquiterpenoid isomers, eudesmanetriol or farnesadiene, which accumulated late in receptacle development.
From the BG stage onward, the achene undergoes a dramatic metabolic shift, with metabolite pools of the major energy pathways being heavily depleted. This presumably fuels storage compound accumulation and fatty acid elongation in a similar manner to the one postulated in seeds (Fait et al., 2006; Junker et al., 2007). In keeping with our results, accumulation of mRNAs related to the buildup of active storage reserves was previously shown to characterize these stages of strawberry development (Aharoni and O'Connell, 2002). Despite the fact that the few fatty acids we measured in this study were maintained at steady levels throughout this second developmental period, the dramatic decrease in CitA is consistent with our hypothesis. In the second half of the mid to late maturation period, other important storage compounds accumulated in the achene (Raf, sorbitol, glycerol, gentiobiose) in a pattern analogous to the metabolic shift detected during maturation of Arabidopsis seeds (Baud et al., 2002; Fait et al., 2006).
In parallel with the changes observed in carbohydrate levels in the achene, an abrupt change in redox status at the ripening stage is apparent, following which both CitA and AscA levels are synchronously reduced. Such a link with regard to cellular redox poise is probably due to the intimate relation of both metabolites to the mitochondrial electron transport chain (Nunes-Nesi et al., 2005; Noctor et al., 2007b).
The secondary metabolism of maturing achenes also exhibited considerable shifts. While, compared with the level monitored in the receptacle, hexose derivatives of several different aromatic acids were low in content, the flavonol derivatives kaempferol hexose and kaempferol coumaroyl hexose accumulated to marked levels at this stage, with an initial dramatic increase at mid maturation. Although these metabolites have been detected previously in strawberry (Aaby et al., 2007a), our results reveal that the biosynthesis of the acylated flavonols occurs mainly in the achene. The acylation of natural products is a phenomenon that is known to alter their polarity, volatility, chemical stability, and, arguably (and most importantly), their biological activity (D'Auria, 2006). Acylation of kaempferol may be related to its protective function or alternatively to its solubility in the achene lipid-rich environment. While it is suggested that differentially substituted flavonoids have different physiological functions (Lim and Bowles, 2004; Bowles et al., 2005), the mechanisms determining partition across the various functional groups is as yet unclear (Winkel, 2004; Jorgensen et al., 2005; Dana et al., 2006).
Correlative Behavior Suggests Developmentally Regulated Cross Talk of Amino Acids, Hormones, and Secondary Metabolism
In general, correlation network analysis suggests that metabolism in the receptacle is not highly coordinated either in early or late stages of development. There is, however, a high degree of coordination of the metabolic changes in the achene, particularly during early development.
When looking into the patterns of change of metabolites across development, subtle associations can be highlighted. In the receptacle, the two-wave behavior of Trp, Phe, and, intriguingly, Gln suggests a synchronous regulation of primary nitrogen metabolism and secondary metabolism. The link between Trp and Gln has been described previously in Arabidopsis (Zhao et al., 1998). The behavior that we report here potentially reflects the need to provide building blocks for the biosynthesis of secondary metabolites of different classes during development, as postulated previously by Zhao and Last (1996). For instance, in our UPLC-QTOF-MS analysis of the receptacle extracts, Phe-derived flavonoids accumulated in the form of proanthocyanidins during the early stages of development, and during the ripening period, the metabolite production shifted toward the end products of the flavonoid pathway, observed as intensive anthocyanin pigmentation. Of direct relevance to this observation, Aharoni et al. (2002) measured a two-phase pattern of expression of the phenylpropanoid and flavonoid pathway genes, being active in the early green stages, decreasing at the white phase, and increasing again during the turning stage of strawberry fruit development. Taking these lines of evidence together, we can suggest that the pattern of change displayed by the shikimate amine precursors and by the nitrogen source Gln in the receptacle could support the developmentally distinct accumulation of different classes of secondary metabolites: tannins during early development and phenolic acids, flavonols, and anthocyanins during ripening. Similarly, the decrease of QuinA measured in the achene, the precursor of chlorogenate and shikimate, and of the closely related aromatic amines Phe, Tyr, and tyramine further suggests high secondary metabolite activity: Phe is a key precursor of phenylpropanoids; tyramine can be converted to defense-related hydroxycinnamic acid tyramine amides (Hohlfeld et al., 1995; Schmidt et al., 1999); QuinA-derived gallic acid is the essential building block of ellagitannins, which we have shown to accumulate particularly in the achene.
Auxin production by the achenes and its transport to the receptacle have been shown to sustain receptacle enlargement in early stages of development (Nitsch, 1950) and to retard fruit ripening (Nitsch, 1950; Given et al., 1988; Cohen, 1996). Consistent with this pattern, we observed that levels of the auxin-related amino acid Trp increased during the initial stages of achene development but displayed a rapid decrease at mid achene maturation. These results suggest that Trp was either strongly catabolized or exported to the receptacle. Further studies, such as metabolic flux analysis, are needed to provide substantiating evidence for this hypothesis.
CONCLUSION
With the aim of providing a global view of the metabolic events associated with fruit development, we here describe, to our knowledge, the first parallel profiling of primary and secondary metabolism of strawberry receptacle and achenes across several developmental stages. Our results reveal that both metabolic synchrony and speciation exist during the development of achenes and receptacle in strawberry fruit (Fig. 9). Changes in metabolite levels in the receptacle most probably reflect the metabolic activity of the fruit. As such, these are somewhat similar to those seen previously in (climacteric) tomato fruit (Carrari et al., 2006), although displaying clear differences, such as the mild accumulation of TCA cycle intermediates. When comparing our results with those of previous studies concerned with the analysis of gene expression (Aharoni and O'Connell, 2002), we were able to suggest that the metabolic program of the achene is largely concerned with the accumulation of storage and protective compounds as well as precursors for hormonal and secondary metabolites. These are required to sustain seed quiescence and secure future germination, to support prolonged pregermination periods, and to guide aspects of seed development, respectively. In addition, we were able to evaluate the coregulation of a vast number of metabolites both across development and between organs. These analyses revealed concerted regulation within and between metabolite categories (e.g. amino acids and TCA cycle intermediates, amino acids, and procyanidin derivatives). Correlation-based network analysis highlighted a dense degree of connectivity, building organ-specific metabolic modules, mainly during early to mid strawberry maturation. Intriguingly, the sugar module in the receptacle correlation network resembled that recently described for tomato pericarp tissue (Ursem et al., 2008). This and other evidence described here suggest a common mechanism between climacteric and nonclimacteric fruit as the basis of metabolic regulation. Taken together, the comprehensive metabolite analysis described here sheds light on the metabolic regulation during strawberry development and the different metabolic programs occurring in two functionally distinct organs. It further highlights the cross talk between primary and secondary metabolism. The conspicuous differences found between receptacle and achenes emphasize the importance of dissecting the organ-specific events in order to gain a better understanding of strawberry growth, development, and response to environmental cues.
Figure 9.
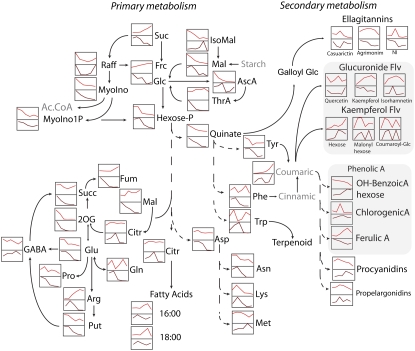
Schematic representation of a number of the major processes in primary and secondary metabolism characterizing the development of receptacle and achenes in strawberry fruit. Changes in metabolism are qualitative. Red line graphs and brown line graphs depict the patterns of change of a specific metabolite in receptacle and achenes, respectively. Amino acids are listed in the conventional three-letter coding. Ac.CoA, Acetyl-CoA; A suffix, acid; Flv, flavonoid; NI, not identified; P suffix, phosphate group; Put, putrescine.
MATERIALS AND METHODS
Plant Material
Frozen strawberries (Fragaria × ananassa) were ground to a fine powder using a grinding mill (analytical mill A11 basic; IKA) or a mortal and pestle. Plants of the Israeli cultivar Herut were grown in a plastic tunnel in the Sharon coastal area in Israel. Fruit were harvested at six different developmental stages (Fig. 1) five times during the season (each harvest served as a replicate). Achenes were removed and collected manually using a scalpel or shaking them frozen in a 50-mL tube (for young fruit).
Standard Compounds and Chemicals for Analytical Chemistry
Unless stated otherwise, all chemicals were purchased either from Sigma-Aldrich or from Merck. Naringenin, chlorogenic acid hemihydrate, and trans-cinnamic acid were purchased from Fluka; ferulic acid was from Aldrich; α-tomatine was from Apin Chemicals; HPLC-grade acetonitrile was from J.T. Baker; methanol was from Merck; formic acid (approximately 98%) was from Fluka. Double deionized water from a Milli-Q purification unit was from Millipore.
Extraction, Derivatization, and Analysis of Polar Metabolites Using GC-MS
Metabolite analysis by GC-MS was carried out essentially as described by Roessner et al. (2001). The chromatograms and mass spectra were evaluated using the MassLab program (ThermoQuest). A retention time and mass spectral library for automatic peak quantification of metabolite derivatives was implemented within the MassLab method. While accurate amino acid determinations have been considered to be problematic using GC-MS technologies (Noctor et al., 2007a), a recent cross-laboratory comparison of the GC-MS protocol working in our institute under the laboratory conditions we routinely use, with traditional HPLC-based methods, suggested that the choice of analytical platform did not unduly influence the results (Böttcher et al., 2008).
Extraction and Analysis of Semipolar Metabolites Using UPLC-QTOF-MS
Frozen powder (200–300 mg) was extracted with 80% (v/v) MeOH, and the solid:liquid ratio was kept at 1:3 (w/v). The mixture was sonicated for 20 min at room temperature, centrifuged (3,000g, 10 min), and filtered through a 0.22-μm PTFE membrane filter (Acrodisc CR 13 mm; PALL) before injection to a UPLC-MS system (Premier QTOF, Waters) with a UPLC column connected online to a UV light detector (Waters Acquity). Separation of metabolites was performed on a 100- × 2.1-mm (i.d.), 1.7-μm UPLC BEH C18 column (Waters Acquity). The mobile phase consisted of 0.1% formic acid in acetonitrile:water (5:95, v/v; phase A) and 0.1% formic acid in acetonitrile (phase B). The linear gradient program was as follows: 100% to 72% A over 22 min, 72% to 60% A over 0.5 min, 60% to 0% A over 0.5 min, hold at 100% B for a further 1.5 min, return to the initial conditions (100% A) in 0.5 min, and conditioning at 100% A for 1 min. The flow rate was 0.3 mL min−1, and the column temperature was kept at 35°C. The UV light trace was measured at 240 nm, and masses were detected by a Q-TOF Premier MS system equipped with an electrospray ionization source. Acquisition was performed in the electrospray ionization-positive and electrospray ionization-negative modes. The following settings were applied during the UPLC-QTOF-MS runs: capillary voltage at 3.0 kV; cone voltage at 30 eV; collision energy at 3 eV; and argon was used as a collision gas. The mass-to-charge ratio (m/z) range was 50 to 1,500 D. The following settings were applied during the UPLC-QTOF-MS/MS runs: capillary spray at 3.0 kV; cone voltage at 30 eV; collision energy at 15 and 30 eV; and argon was used as a collision gas. The MS apparatus was calibrated using sodium formate, and Leu enkaphalin was used as the lock mass. MassLynx software version 4.1 was used to control all instruments and calculate accurate mass. In some cases, the UV light spectra (recorded between 200 and 600 nm) were acquired on another UPLC Waters Acquity instrument equipped with the Acquity 2996 PDA detector and a 150- × 2.1-mm (i.d.), 1.7-μm UPLC BEH C18 column (Waters Acquity). The liquid chromatography conditions were as described for UPLC-QTOF analysis. A mixture of 15 standard compounds, injected after each 10 samples, was used for quality control.
Identification of Metabolites Detected by UPLC-QTOF-MS
Metabolites were identified using standard compounds by comparison of their retention times and MS/MS fragments and putatively identified applying several steps. The elemental composition was selected according to the accurate masses and the isotopic pattern using MassLynx software and searched against metabolite databases: Dictionary of Natural Products (Chapman and Hall/CRC), SciFinder (SciFinder Scholar 2007), the KNApSAcK database (http://prime.psc.riken.jp/KNApSAcK), and the MOTO database (http://appliedbioinformatics.wur.nl/moto). Predicted log D values for pH 3 (pH of the UPLC mobile phase), found using the SciFinder tool, were utilized for the retention time prediction in order to narrow the number of proposed structures. The interpretation of the observed MS/MS spectra in comparison with those found in the literature (where possible) was the main tool for putative identification of metabolites. Some compounds reported earlier in strawberry are annotated based on the fragmentation in source, and in several cases these were analyzed by MS/MS in our previous study (K. Hanhineva, I. Rogachev, H. Kokko, S. Mintz-Oron, I. Venger, S. Kärenlampi, and A. Aharoni, unpublished data). Two of the compounds, eutigoside A and grayanoside A, were identified by NMR in strawberry in our previous study (K Hanhineva and S Kärenlampi, unpublished data). The references to previous liquid chromatography-mass spectrometry analyses of strawberry for each compound are given in Supplemental Table S3.
The metabolites not previously detected in strawberry were putatively identified as follows. Eriodictyol hexose was identified based on MS/MS analysis and was distinguishable from another flavanone, dihydrokaempferol, having the same molecular formula (C15H12O6). The compound exhibited the same MS/MS fragmentation as eriodictyol aglycone as described by Es-Safi et al. (2005; Supplemental Table S3). Naringenin/naringenin chalcone hexose was identified by comparison with the naringenin chalcone standard aglycone. By following the formula [M-H+288]− or [M-2H+144]2− (Nunez et al., 2006), procyanidin polymers up to dekamer were identifiable (Supplemental Table S3; Supplemental Fig. S1) in an additional analysis performed with 4,000 atomic mass unit acquisition (Supplemental Fig. S1). Trace amounts of mass signals corresponding to molecules as large as undekamer (11 units, [1584-2H]2−), dodekamer (12 units, [1728-2H]2−), and tridekamer (13 units, [1872-2H]2−) were observed at the end of the proanthocyanidin elution region at retention times of 8 to 9 min (Supplemental Fig. S2). Following the identification of typical ellagitannin compounds, several peaks possessing ellagitannin characteristics were also found that have not been described in strawberry elsewhere. The characteristic ellagitannin fragmentation is shown in Supplemental Figure S3 with a compound tentatively identified as lambertiain C. Highly tentative annotation could be done to several ellagitannin-like peaks; a single charged ion of m/z 949.06 is the most abundant signal at a retention time of 11.8 min and suggested the molecular formula C41H26O27. A search using the Dictionary of Natural Products database resulted in one ellagitannin, lagerstannin B (CAS 147666-67-9). At retention times of 7.4 and 10.3 min, a compound elutes with m/z 933.06, suggesting the formula C41H26O27, one oxygen smaller than lagerstannin B. Such a formula matches castalagin (CAS 115166-32-0). For both of the peaks mentioned here, there is a corresponding compound with one additional galloyl moiety (+152) with m/z 1,101.07 and 1,085.08, which stand for C48H30O31 and C48H30O30, respectively. These molecular formulae match with compounds castavaloninic acid (CAS 60102-67-2) and galloylcastalagin (CAS 108449-60-1), respectively. At retention time of 7.8 min with m/z 799.06 was a galloyl moiety smaller than lagerstannin B with the suggested elemental composition C34H24O23 and one hit in the Dictionary of Natural Products: lagerstannin A. In addition to these mentioned compounds, there were approximately 10 additional peaks in the achene samples that could bear ellagitannin compounds.
The quantification of the compounds is based on the relative peak response area of each signal in mass chromatograms. The five replicate samples were prepared from the same amount of frozen material, and the results were normalized to the dry weight in both achenes and receptacle. The data presented here are the direct amounts of peak area, as no quantitative standard was applied in this study. The heat map analysis was done on a Microsoft Excel-implemented macro program available at http://www.senorjosh.com/archives/2003/04/heatmap_tool_vba.shtml.
Statistical Analysis of GC-MS and UPLC-QTOF-MS Data
Principal component analysis was performed on the data sets obtained from metabolite profiling with the software package TMEV (Saeed et al., 2003) using the default weighted covariance estimation function. The data were log transformed and normalized to the median of the entire sample set for each metabolite before analysis. This transformation reduces the influence of outliers. Heat map presentation and clustering were performed with Pearson correlation coefficient matrices, and their presentation as heat maps was performed in the R environment for statistical computing (http://www.R-project.org; R Development Core Team, 2004). ANOVA was performed using TMEV software. The term “significant” is used in the text only when the change in question has been confirmed to be significant (P < 0.05, at least). The t tests were performed using the algorithm embedded into Microsoft Excel. A network of metabolite correlations was reconstructed based on the method described by Nikiforova et al. (2005), with the connectivity threshold fixed at the absolute level of Pearson correlation coefficient |R| > 0.9.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Reconstructed ion chromatograms of condensed tannin polymers.
Supplemental Figure S2. Procyanidin pentamer MS spectrum.
Supplemental Figure S3. Ellagitannin MS spectrum (A), tentatively identified as lambertiain C, with typical ellagitannin fragmentation marked in the spectrum (B).
Supplemental Table S1. Principal component analysis and ANOVA P values expressing the statistical significance of the changes in primary metabolite contents across developmental stages.
Supplemental Table S2. Principal component analysis and ANOVA P values expressing the statistical significance of the changes in secondary metabolite contents across developmental stages.
Supplemental Table S3. Tentatively identified secondary metabolites and their abundance in different developmental stages of strawberry receptacle and achenes.
Supplemental Table S4. Pearson correlation coefficients between metabolites throughout developmental stages in strawberry receptacle.
Supplemental Table S5. Pearson correlation coefficients between metabolites throughout developmental stages in strawberry achenes.
Supplemental Table S6. Degree of connectivity of individual metabolites in receptacle and achenes.
Supplementary Material
Acknowledgments
We thank Luigi Cattivelli for his support.
This work was supported by the Alexander von Humboldt Foundation (award to A.F.), by the Saastamoinen Foundation (research visit funding to K.H.), by the Israel Ministry of Science (IMOS grant no. 3–2552 to A.A.), by the European Union project META-PHOR (contract no. FOODCT–2006–036220), by Mr. and Mrs. Mordechai Segal, and by the Henry S. and Anne Reich Family Foundation. A.A. is an incumbent of the Adolfo and Evelyn Blum Career Development Chair.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Aaron Fait (fait@bgu.ac.il).
Some figures in this article are displayed in color online but in black and white in the print edition.
The online version of this article contains Web-only data.
References
- Aaby K, Ekeberg D, Skrede G (2007. a) Characterization of phenolic compounds in strawberry (Fragaria x ananassa) fruits by different HPLC detectors and contribution of individual compounds to total antioxidant capacity. J Agric Food Chem 55 4395–4406 [DOI] [PubMed] [Google Scholar]
- Aaby K, Skrede G, Wrolstad RE (2005) Phenolic composition and antioxidant activities in flesh and achenes of strawberries (Fragaria ananassa). J Agric Food Chem 53 4032–4040 [DOI] [PubMed] [Google Scholar]
- Aaby K, Wrolstadt RE, Ekeberg D, Skrede G (2007. b) Polyphenol composition and antioxidant activity in strawberry purees: impact of achene level and storage. J Agric Food Chem 55 5156–5166 [DOI] [PubMed] [Google Scholar]
- Aharoni A, Giri AP, Verstappen FW, Bertea CM, Sevenier R, Sun Z, Jongsma MA, Schwab W, Bouwmeester HJ (2004) Gain and loss of fruit flavor compounds produced by wild and cultivated strawberry species. Plant Cell 16 3110–3131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharoni A, Keizer LC, Van Den Broeck HC, Blanco-Portales R, Muñoz-Blanco J, Bois G, Smit P, De Vos RC, O'Connell AP (2002. a) Novel insight into vascular, stress, and auxin-dependent and -independent gene expression programs in strawberry, a non-climacteric fruit. Plant Physiol 129 1019–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharoni A, O'Connell AP (2002) Gene expression analysis of strawberry achene and receptacle maturation using DNA microarrays. J Exp Bot 53 2073–2087 [DOI] [PubMed] [Google Scholar]
- Aharoni A, Ric de Vos CH, Verhoeven HA, Maliepaard CA, Kruppa G, Bino R, Goodenowe DB (2002. b) Nontargeted metabolome analysis by use of Fourier transform ion cyclotron mass spectrometry. OMICS 6 217–234 [DOI] [PubMed] [Google Scholar]
- Almeida JRM, D'Amico E, Preuss A, Carbone F, de Vos CHR, Deiml B, Mourgues F, Perrotta G, Fischer TC, Bovy AG, et al (2007) Characterization of major enzymes and genes involved in flavonoid and proanthocyanidin biosynthesis during fruit development in strawberry (Fragaria x ananassa). Arch Biochem Biophys 465 61–71 [DOI] [PubMed] [Google Scholar]
- Baud S, Boutin JP, Miquel M, Lepiniec L, Rochat C (2002) An integrated overview of seed development in Arabidopsis thaliana ecotype WS. Plant Physiol Biochem 40 151–160 [Google Scholar]
- Bedair M, Sumner LW (2008) Current and emerging mass-spectrometry technologies for metabolomics. Trends Analyt Chem 27 238–250 [Google Scholar]
- Benítez-Burraco A, Blanco-Portales R, Redondo-Nevado J, Bellido ML, Moyano E, Caballero JL, Muñoz-Blanco J (2003) Cloning and characterization of two ripening-related strawberry (Fragaria x ananassa cv. Chandler) pectate lyase genes. J Exp Bot 54 633–645 [DOI] [PubMed] [Google Scholar]
- Besseau S, Hoffmann L, Geoffroy P, Lapierre C, Pollet B, Legrand M (2007) Flavonoid accumulation in Arabidopsis repressed in lignin synthesis affects auxin transport and plant growth. Plant Cell 19 148–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bewley JD, Black M (1994) Seeds: Physiology of Development and Germination, Ed 2. Plenum Press, NY
- Blanco-Portales R, Medina-Escobar N, López-Ráez JA, González-Reyes JA, Villalba JM, Moyano E, Caballero JL, Muñoz-Blanco J (2002) Cloning, expression and immunolocalization pattern of a cinnamyl alcohol dehydrogenase gene from strawberry (Fragaria x ananassa cv. Chandler). J Exp Bot 53 1723–1734 [DOI] [PubMed] [Google Scholar]
- Borisjuk L, Rolletschek H, Radchuk R, Weschke W, Wobus U, Weber H (2004) Seed development and differentiation: a role for metabolic regulation. Plant Biol 6 375–386 [DOI] [PubMed] [Google Scholar]
- Böttcher C, Centeno D, Freitag J, Höfgen R, Köhl K, Kopka J, Kroymann J, Matros A, Mock HP, Neumann S, et al (2008) Teaching (and learning from) metabolomics: the 2006 PlantMetaNet ETNA Metabolomics Research School. Physiol Plant 132 136–149 [DOI] [PubMed] [Google Scholar]
- Bowles D, Isayenkova J, Lim EK, Poppenberger B (2005) Glycosyltransferases: managers of small molecules. Curr Opin Plant Biol 8 254–263 [DOI] [PubMed] [Google Scholar]
- Camacho DM (2005) The origin of correlations in metabolomics data. Metabolomics 1 53–63 [Google Scholar]
- Carrari F, Baxter C, Usadel B, Urbanczyk-Wochniak E, Zanor MI, Nunes-Nesi A, Nikiforova V, Centero D, Ratzka A, Pauly M, et al (2006) Integrated analysis of metabolite and transcript levels reveals the metabolic shifts that underlie tomato fruit development and highlight regulatory aspects of metabolic network behavior. Plant Physiol 142 1380–1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrari F, Fernie AR (2006) Metabolic regulation underlying tomato fruit development. J Exp Bot 57 1883–1987 [DOI] [PubMed] [Google Scholar]
- Castillejo C, de la Fuente JI, Iannetta P, Botella MA, Valpuesta V (2004) Pectin esterase gene family in strawberry fruit: study of FaPE1, a ripening-specific isoform. J Exp Bot 55 909–918 [DOI] [PubMed] [Google Scholar]
- Cheng GW, Breen PJ (1991) Activity of phenylalanine ammonia-lyase (PAL) and concentrations of anthocyanins and phenolics in developing strawberry fruit. J Am Soc Hortic Sci 116 865–869 [Google Scholar]
- Cheng GW, Breen PJ (1992) Cell count and size in relation to fruit size among strawberry cultivars. J Am Soc Hortic Sci 117 946–950 [Google Scholar]
- Cohen JD (1996) In vitro tomato fruit cultures demonstrate a role for indole-3-acetic acid in regulating fruit ripening. J Am Soc Hortic Sci 121 520–524 [Google Scholar]
- Corbesier L, Vincent C, Jang S, Fornara F, Fan Q, Searle I, Giakountis A, Farrona S, Gissot L, Turnbull C, Coupland G (2007) FT protein movement contributes to long-distance signaling in floral induction of Arabidopsis. Science 316 1030–1033 [DOI] [PubMed] [Google Scholar]
- Dana CD, Bevan DR, Winkel BSJ (2006) Molecular modeling of the effects of mutant alleles on chalcone synthase protein structure. J Mol Model 12 905–914 [DOI] [PubMed] [Google Scholar]
- D'Auria JC (2006) Acyltransferases in plants: a good time to be BAHD. Curr Opin Plant Biol 9 331–340 [DOI] [PubMed] [Google Scholar]
- Debolt S, Melino V, Ford CM (2007) Ascorbate as a biosynthetic precursor in plants. Ann Bot (Lond) 99 3–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng C, Davis TM (2001) Molecular identification of the yellow fruit color (c) locus in diploid strawberry: a candidate gene approach. Theor Appl Genet 103 316–322 [Google Scholar]
- Dixon RA, Paiva NL (1995) Stress-induced phenylpropanoid metabolism. Plant Cell 7 1085–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon RA, Xie DY, Sharma SB (2005) Proanthocyanidins: a final frontier in flavonoid research? New Phytol 165 9–28 [DOI] [PubMed] [Google Scholar]
- Fait A, Angelovici R, Less H, Ohad I, Urbanczyk-Wochniak E, Fernie AR, Galili G (2006) Arabidopsis seed development and germination is associated with temporally distinct metabolic switches. Plant Physiol 142 839–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forney CF, Breen PJ (1985) Growth of strawberry fruit and sugar uptake of fruit disks at different inflorescence positions. Sci Hortic (Amsterdam) 27 55–62 [Google Scholar]
- Forney CF, Breen PJ (1986) Sugar content and uptake in the strawberry fruit. J Am Soc Hortic Sci 111 241–247 [Google Scholar]
- Fossen T, Rayyan S, Andersen ØM (2004) Dimeric anthocyanins from strawberry (Fragaria ananassa) consisting of pelargonidin 3-glucoside covalently linked to four flavan-3-ols. Phytochemistry 65 1421–1428 [DOI] [PubMed] [Google Scholar]
- Gatz SA, Wiesmüller L (2008) Take a break: resveratrol in action on DNA. Carcinogenesis 29 321–332 [DOI] [PubMed] [Google Scholar]
- Gerwin B, Burstein SR, Westley J (1974) Ascorbate oxidase: inhibition, activation, and pH effects. J Biol Chem 249 2005–2008 [PubMed] [Google Scholar]
- Gibson SI (2005) Control of plant development and gene expression by sugar signalling. Curr Opin Plant Biol 8 93–102 [DOI] [PubMed] [Google Scholar]
- Gillaspy G, Ben-David H, Gruissem W (1993) Fruits: a developmental perspective. Plant Cell 5 1439–1451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Given NK, Venis MA, Grierson D (1988) Hormonal-regulation of ripening in the strawberry, a non-climacteric fruit. Planta 174 402–406 [DOI] [PubMed] [Google Scholar]
- Gomez-Merino FC, Arana-Ceballos FA, Trejo-Tellez LI, Skirycz A, Brearley CA, Dormann P, Mueller-Roeber B (2005) Arabidopsis AtDGK7, the smallest member of plant diacylglycerol kinases (DGKs), displays unique biochemical features and saturates at low substrate concentration: the DGK inhibitor R59022 differentially affects AtDGK2 and AtDGK7 activity in vitro and alters plant growth and development. J Biol Chem 280 34888–34899 [DOI] [PubMed] [Google Scholar]
- Green MA, Fry SC (2005) Vitamin C degradation in plant cells via enzymatic hydrolysis of 4-O-oxalyl-L-threonate. Nature 433 83–87 [DOI] [PubMed] [Google Scholar]
- Gross M (2007) Molecular trees bear fruit. Chemistry World 4 62–66 [Google Scholar]
- Gu LW, Kelm MA, Hammerstone JF, Beecher G, Holden J, Haytowitz D, Prior RL (2003) Screening of foods containing proanthocyanidins and their structural characterization using LC-MS/MS and thiolytic degradation. J Agric Food Chem 51 7513–7521 [DOI] [PubMed] [Google Scholar]
- Halbwirth H, Puhl I, Haas U, Jezik K, Treutter D, Stich K (2006) Two-phase flavonoid formation in developing strawberry (Fragaria x ananassa) fruit. J Agric Food Chem 54 1479–1485 [DOI] [PubMed] [Google Scholar]
- Hancock JF (2000) Strawberries. In A Erez, ed, Temperate Fruit Crops in Warm Climates. Kluwer Academic Publishers, Dordrecht, The Netherlands
- Haslam E, Cai Y (1994) Plant polyphenols (vegetable tannins): gallic acid metabolism. Nat Prod Rep 11 41–66 [DOI] [PubMed] [Google Scholar]
- Havis AL (1943) A developmental analysis of the strawberry fruit. Am J Bot 30 311–314 [Google Scholar]
- Hohlfeld H, Schurmann W, Scheel D, Strack D (1995) Partial purification and characterization of hydroxycinnamoyl-coenzyme A-tyramine hydroxycinnamoyltransferase from cell-suspension cultures of Solanum tuberosum. Plant Physiol 107 545–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hukkanen AT, Kokko HI, Buchala AJ, McDougall GJ, Stewart D, Kärenlampi SO, Karjalainen RO (2007) Benzothiadiazole induces the accumulation of phenolics and improves resistance to powdery mildew in strawberries. J Agric Food Chem 55 1862–1870 [DOI] [PubMed] [Google Scholar]
- Humphrey JM, Chapple C (2002) Rewriting the lignin roadmap. Curr Opin Plant Biol 5 224–229 [DOI] [PubMed] [Google Scholar]
- Jones-Rhoades MW, Bartel DP, Bartel B (2006) MicroRNAs and their regulatory roles in plants. Annu Rev Plant Biol 57 19–53 [DOI] [PubMed] [Google Scholar]
- Jorgensen BB, Glud RN, Holby O (2005) Oxygen distribution and bioirrigation in Arctic fjord sediments (Svalbard, Barents Sea). Mar Ecol Prog Ser 292 85–95 [Google Scholar]
- Junker BH, Lonien J, Heady LE, Rogers A, Schwender J (2007) Parallel determination of enzyme activities and in vivo fluxes in Brassica napus embryos grown on organic or inorganic nitrogen source. Phytochemistry 68 2232–2242 [DOI] [PubMed] [Google Scholar]
- Kalantidis K, Schumacher HT, Alexiadis T, Helm JM (2008) RNA silencing movement in plants. Biol Cell 100 13–26 [DOI] [PubMed] [Google Scholar]
- Knee M, Sargent JA, Osborne DJ (1977) Cell-wall metabolism in developing strawberry fruits. J Exp Bot 28 377–396 [Google Scholar]
- Landmann C, Fink B, Schwab W (2007) FaGT2: a multifunctional enzyme from strawberry (Fragaria x ananassa) fruits involved in the metabolism of natural and xenobiotic compounds. Planta 226 417–428 [DOI] [PubMed] [Google Scholar]
- Lepiniec L, Debeaujon I, Routaboul JM, Baudry A, Pourcel L, Nesi N, Caboche M (2006) Genetics and biochemistry of seed flavonoids. Annu Rev Plant Biol 57 405–430 [DOI] [PubMed] [Google Scholar]
- Lim EK, Bowles DJ (2004) A class of plant glycosyltransferases involved in cellular homeostasis. EMBO J 23 2915–2922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey K (2001) Plant peptide hormones: the long and the short of it. Curr Biol 11 R741–R743 [DOI] [PubMed] [Google Scholar]
- Lunkenbein S, Bellido M, Aharoni A, Salentijn EMJ, Kaldenhoff R, Coiner HA, Munoz-Blanco J, Schwab W (2006. a) Cinnamate metabolism in ripening fruit: characterization of a UDP-glucose:cinnamate glucosyltransferase from strawberry. Plant Physiol 140 1047–1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunkenbein S, Coiner H, de Vos CHR, Schaart JG, Boone MJ, Krens FA, Schwab W, Salentijn EMJ (2006. b) Molecular characterization of a stable antisense chalcone synthase phenotype in strawberry (Fragaria x ananassa). J Agric Food Chem 54 2145–2153 [DOI] [PubMed] [Google Scholar]
- Lunkenbein S, Salentijn EMJ, Coiner HA, Boone MJ, Krens FA, Schwab W (2006. c) Up- and down-regulation of Fragariaxananassa O-methyltransferase: impacts on furanone and phenylpropanoid metabolism. J Exp Bot 57 2445–2453 [DOI] [PubMed] [Google Scholar]
- Määttä-Riihinen KR, Kamal-Eldin A, Torronen AR (2004) Identification and quantification of phenolic compounds in berries of Fragaria and Rubus species (family Rosaceae). J Agric Food Chem 52 6178–6187 [DOI] [PubMed] [Google Scholar]
- Macías-Rodríguez L, Quero E, López MG (2002) Carbohydrate differences in strawberry crowns and fruit (Fragaria x ananassa) during plant development. J Agric Food Chem 50 3317–3321 [DOI] [PubMed] [Google Scholar]
- Manning K (1994) Changes in gene-expression during strawberry fruit ripening and their regulation by auxin. Planta 194 62–68 [Google Scholar]
- Manning K (1998) Isolation of a set of ripening-related genes from strawberry: their identification and possible relationship to fruit quality traits. Planta 205 622–631 [DOI] [PubMed] [Google Scholar]
- Mo YY, Nagel C, Taylor LP (1992) Biochemical complementation of chalcone synthase mutants defines a role for flavonols in functional pollen. Proc Natl Acad Sci USA 89 7213–7217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moing A, Renaud C, Gaudillere M, Raymond P, Roudeillac P, Denoyes-Rothan B (2001) Biochemical changes during fruit development of four strawberry cultivars. J Am Soc Hortic Sci 126 394–403 [Google Scholar]
- Mullen W, Yokota T, Lean MEJ, Crozier A (2003) Analysis of ellagitannins and conjugates of ellagic acid and quercetin in raspberry fruits by LC-MSn. Phytochemistry 64 617–624 [DOI] [PubMed] [Google Scholar]
- Nikiforova VJ, Daub CO, Hesse H, Willmitzer L, Hoefgen R (2005) Integrative gene-metabolite network with implemented causality deciphers informational fluxes of sulfur stress response. J Exp Bot 56 1887–1896 [DOI] [PubMed] [Google Scholar]
- Nitsch JP (1950) Growth and morphogenesis of the strawberry as related to auxin. Am J Bot 37 211–215 [Google Scholar]
- Noctor G, Bergot G, Mauve C, Thominet D, Lelarge-Trouverie C, Prioulet JL (2007. a) A comparative study of amino acid measurement in leaf extracts by gas chromatography-time of flight-mass spectrometry and high performance liquid chromatography with fluorescence detection. Metabolomics 3 161–174 [Google Scholar]
- Noctor G, Dutilleul C, Lelarge C, Prioul J, De Paepe R, Foyer C (2007. b) The role of the mitochondrial electron transport chain in photosynthesis, stress responses, and the integration of carbon-nitrogen metabolism. Photosynth Res 91 259–259 [Google Scholar]
- Nunes-Nesi A, Carrari F, Lytovchenko A, Smith AMO, Loureiro ME, Ratcliffe RG, Sweetlove LJ, Fernie AR (2005) Enhanced photosynthetic performance and growth as a consequence of decreasing mitochondrial malate dehydrogenase activity in transgenic tomato plants. Plant Physiol 137 611–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez V, Gomez-Cordoves C, Bartolome B, Hong YJ, Mitchell AE (2006) Non-galloylated and galloylated proanthocyanidin oligomers in grape seeds from Vitus vinifera L. cv. Graciano, Tempranillo and Cabernet Sauvignon. J Sci Food Agric 86 915–921 [Google Scholar]
- Osbourn AE (2003) Saponins in cereals. Phytochemistry 62 1–4 [DOI] [PubMed] [Google Scholar]
- Park YS, Jung ST, Kang SG, Drzewiecki J, Namiesnik J, Haruenkit R, Barasch D, Trakhtenberg S, Gorinstein S (2006) In vitro studies of polyphenols, antioxidants and other dietary indices in kiwifruit (Actinidia deliciosa). Int J Food Sci Nutr 57 107–122 [DOI] [PubMed] [Google Scholar]
- Perez AG, Olias R, Olias JM, Sanz C (1999) Biosynthesis of 4-hydroxy-2,5-dimethyl-3(2H)-furanone and derivatives in in vitro grown strawberries. J Agric Food Chem 47 655–658 [DOI] [PubMed] [Google Scholar]
- Perkins-Veazie P (1995) Growth and ripening of strawberry fruit. Hortic Rev (Am Soc Hortic Sci) 17 267–297 [Google Scholar]
- R Development Core Team (2004) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna
- Roessner U, Luedemann A, Brust D, Fiehn O, Linke T, Willmitzer L, Fernie AR (2001) Metabolic profiling allows comprehensive phenotyping of genetically or environmentally modified plant systems. Plant Cell 13 11–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roessner-Tunali U, Hegemann B, Lytovchenko A, Carrari F, Bruedigam C, Granot D, Fernie AR (2003) Metabolic profiling of transgenic tomato plants overexpressing hexokinase reveals that the influence of hexose phosphorylation diminishes during fruit development. Plant Physiol 133 84–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeed AI, Sharov V, White J, Li J, Liang W, Bhagabati N, Braisted J, Klapa M, Currier T, Thiagarajan M, et al (2003) TM4: a free, open-source system for microarray data management and analysis. Biotechniques 34 374–378 [DOI] [PubMed] [Google Scholar]
- Schauer N, Semel Y, Roessner U, Gur A, Balbo I, Carrari F, Pleban T, Perez-Melis A, Bruedigam C, Kopka J, et al (2006) Comprehensive metabolic profiling and phenotyping of interspecific introgression lines for tomato improvement. Nat Biotechnol 24 447–454 [DOI] [PubMed] [Google Scholar]
- Schmidt A, Grimm R, Schmidt J, Scheel D, Strack D, Rosahl S (1999) Cloning and expression of a potato cDNA encoding hydroxycinnamoyl-CoA:tyramine N-(hydroxycinnamoyl)transferase. J Biol Chem 274 4273–4280 [DOI] [PubMed] [Google Scholar]
- Sparg SG, Light ME, van Staden J (2004) Biological activities and distribution of plant saponins. J Ethnopharmacol 94 219–243 [DOI] [PubMed] [Google Scholar]
- Steuer R (2006) On the analysis and interpretation of correlations in metabolomic data. Brief Bioinform 7 151–158 [DOI] [PubMed] [Google Scholar]
- Suutarinen J, Anakainen L, Autio K (1998) Comparison of light microscopy and spatially resolved Fourier transform infrared (FT-IR) microscopy in the examination of cell wall components of strawberries. Lebensm Wiss Technol 31 595–601 [Google Scholar]
- Tamaki S, Matsuo S, Wong HL, Yokoi S, Shimamoto K (2007) Hd3a protein is a mobile flowering signal in rice. Science 316 1033–1036 [DOI] [PubMed] [Google Scholar]
- Ursem R, Tikunov Y, Bovy A, van Berloo R, van Eeuwijk F (2008) A correlation network approach to metabolic data analysis for tomato fruits. Euphytica 161 181–193 [Google Scholar]
- Valpuesta V, Botella MA (2004) Biosynthesis of L-ascorbic acid in plants: new pathways for an old antioxidant. Trends Plant Sci 9 573–577 [DOI] [PubMed] [Google Scholar]
- Wang SY, Chen CT, Wang CY, Chen P (2007) Resveratrol content in strawberry fruit is affected by preharvest conditions. J Agric Food Chem 55 8269–8274 [DOI] [PubMed] [Google Scholar]
- Weber H, Borisjuk L, Wobus U (2005) Molecular physiology of legume seed development. Annu Rev Plant Biol 56 253–279 [DOI] [PubMed] [Google Scholar]
- Wein M, Lavid N, Lunkenbein S, Lewinsohn E, Schwab W, Kaldenhoff R (2002) Isolation, cloning and expression of a multifunctional O-methyltransferase capable of forming 2,5-dimethyl-4-methoxy-3(2H)-furanone, one of the key aroma compounds in strawberry fruits. Plant J 31 755–765 [DOI] [PubMed] [Google Scholar]
- Winkel BS (2004) Metabolic channeling in plants. Annu Rev Plant Biol 55 85–107 [DOI] [PubMed] [Google Scholar]
- Zhao JM, Last RL (1996) Coordinate regulation of the tryptophan biosynthetic pathway and indolic phytoalexin accumulation in Arabidopsis. Plant Cell 8 2235–2244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao JM, Williams CC, Last RL (1998) Induction of Arabidopsis tryptophan pathway enzymes and camalexin by amino acid starvation, oxidative stress, and an abiotic elicitor. Plant Cell 10 359–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.