In general, the basic principles of trafficking systems in plant cells appear to be similar to those in animal and yeast cells, indicating that trafficking mechanisms are highly conserved throughout all eukaryotes (Jurgens, 2004; Vitale and Hinz, 2005). However, there are still considerable variations at the molecular level as well as in applying these basic principles to situations in plant cells, such as multiple vacuole types in a single cell or cell plate formation during cytokinesis (Jurgens, 2004; Jolliffe et al., 2005; Robinson et al., 2005, 2007; Mo et al., 2006). Additionally, the role of the trans-Golgi network (TGN) as the early endosome in plant cells is drastically different from the function of the TGN in animal cells and yeast (Lam et al., 2007). These differences provide compelling reasons to study protein trafficking in plant cells. Earlier studies have focused primarily on the identification of sorting motifs in cargo proteins. However, recent studies have focused more on identification of the molecular players involved in the various steps of trafficking (Jurgens, 2004; Robinson et al., 2007). Detailed studies of the components of these pathways will provide a deeper understanding of these important aspects of plant biology and may elucidate similarities, as well as differences, in trafficking mechanisms between animal and plant cells.
THE ANTEROGRADE TRAFFICKING PATHWAYS AT THE GOLGI APPARATUS
Newly synthesized organellar proteins are delivered to their respective organelles by a complex mechanism of transport. Secretory proteins, along with proteins destined for the central lytic vacuole, protein storage vacuole (PSV), plasma membrane, or endosomes, are initially sorted at translation when they are cotranslationally translocated into the endoplasmic reticulum (ER; Crowley et al., 1994; Rapoport et al., 1996). After correct folding and assembly in the ER, the majority of these proteins are transported to the Golgi complex by coat protein II vesicles (Tang et al., 2005). These proteins are then subjected to sorting, primarily at the Golgi apparatus, depending on their final destinations (Marcusson et al., 1994; Hadlington and Denecke, 2000; Gu et al., 2001; Jurgens, 2004). The final destinations include the prevacuolar compartment (PVC), central lytic vacuole, PSV, plasma membrane, apoplastic space, and endosomes.
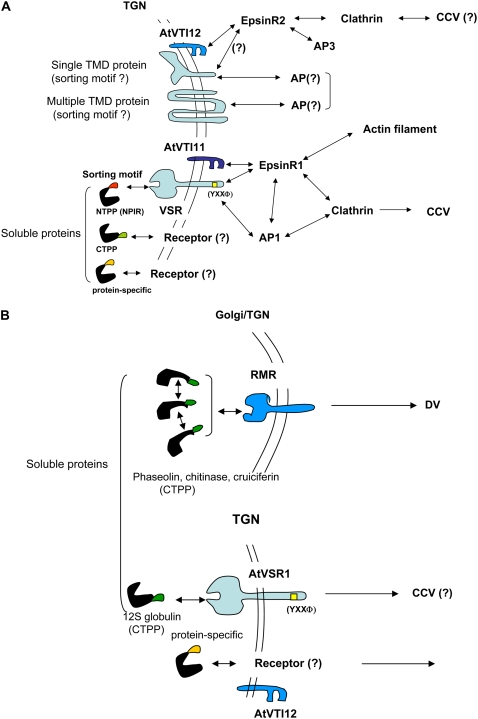
The Golgi apparatus plays a pivotal role in the sorting of proteins destined for various post-Golgi compartments (Jurgens, 2004; Mo et al., 2006; Hanton et al., 2007; Lam et al., 2007). Sorting of proteins destined for the central lytic vacuole occurs at the TGN and appears to be similar to sorting to the lysosome and the vacuole in animal cells and yeast, respectively. In animal cells and yeast, proteins sorted at the TGN are transported to the lysosome/vacuole by multiple routes (Nakatsu and Ohno, 2003; Bowers and Stevens, 2005; Newell-Litwa et al., 2007). In one route, proteins are delivered to the late endosome in animal cells or the PVC in yeast before they are finally transferred to the lysosome/vacuole. The majority of soluble cargo proteins appear to take this route. Among proteins transported by this route, the best characterized proteins include carboxypeptidase Y in yeast and soluble hydrolases with Man-6-P-containing glycans in animal cells (Sly and Fisher, 1982; Jung et al., 1999). In contrast, membrane proteins such as alkaline phosphatase in yeast and lysosomal integral membrane protein-II (LIMP-II) in animal cells are transported to the vacuole/lysosome by a different route (Piper et al., 1997; Honing et al., 1998; Robinson, 2004). The specific transport pathways taken by cargo proteins depend on the sorting motifs carried by the proteins (Gu et al., 2001; Robinson, 2004; Ni et al., 2006; Piper and Luzio, 2007). In plant cells, soluble cargo proteins that are recognized by BP80-type vacuolar sorting receptors (VSRs) at the TGN are transported to the PVC by clathrin-coated vesicles (CCVs) before they are transported to the vacuole (Fig. 1A). Whether additional routes for proteins destined for the central lytic vacuole exist in plant cells has not been clearly demonstrated. One possibility is that tonoplast membrane proteins may be transported by a route that differs from vacuole luminal proteins, as observed in yeast and animal cells (Odorizzi et al., 1998; Bonifacino and Traub, 2003; Robinson, 2004; Baust et al., 2008). Indeed, the presence of an additional TGN-to-vacuole route is supported by multiple lines of indirect evidence, including the presence of the heterotetrameric adaptor protein-3 (AP-3) and the monomeric adaptor EpsinR2 (see below for details; Fig. 1A; Lee et al., 2007a).
Figure 1.
A working model of the sorting of proteins destined for the central lytic vacuole and PSV from the Golgi apparatus and TGN. A, Sorting of proteins to the central lytic vacuole. Proteins containing the NPIR-type sorting motif are sorted by VSRs of the BP80 family. VSRs may interact with the adaptor proteins AP-1 and/or EpsinR1. AP-1 and EpsinR1 may interact with each other and with clathrin in the formation of CCVs. EpsinR1 interacts with AtVTI11 and actin filaments. This network of interactions between proteins may facilitate the formation of CCVs by efficiently recruiting accessory proteins to the TGN. In contrast, cargo receptors for proteins with CTPP or protein-specific sorting motifs are unknown. Furthermore, the adaptors of membrane proteins destined for the PVC and the lytic vacuole are largely unknown in plant cells. EpsinR2, which interacts with AtVTI12, AP-3, and clathrin, may also play a role in the trafficking of protein cargoes through CCVs. B, Sorting of proteins to the PSV. Proteins start to aggregate in the cis-Golgi and continue aggregating throughout the Golgi stacks until they bud off at the TGN as dense vesicles. RMR functions as a sorting receptor for the PSV. In the process of PSV protein aggregation through the Golgi stacks, it is not clear when RMR is involved in the recognition of PSV proteins. In addition, proteins may be sorted at the TGN to the PSV by the sorting receptor AtVSR1. AtVTI12 has been shown to play a role in the PSV pathway. However, it is not clear at present how it functions at the TGN. DV, Dense vesicle.
Seed storage proteins are also transported from the Golgi apparatus to the PSV (Jiang and Rogers, 1998; Jiang et al., 2000; Robinson et al., 2005; Vitale and Hinz, 2005). The sorting mechanism of the abundant PSV proteins at the Golgi apparatus appears to be different from that of central vacuolar proteins. The detailed mechanism of protein targeting to the PSV has been studied by electron microscopy in seed cells (Jiang and Rogers, 1998; Hinz et al., 1999, 2007; Hillmer et al., 2001). The PSV proteins start to aggregate in the cis-Golgi, and continued aggregation occurs throughout the Golgi stacks until the proteins bud off at the TGN in dense vesicles (Hillmer et al., 2001; Hinz et al., 2007). After leaving the TGN, PSV proteins may also be transported to the PSV through the PVC/multivesicular body (Otegui et al., 2006; Hinz et al., 2007; Wang et al., 2007). Despite detailed observations of the sorting of PSV proteins at the Golgi stacks by electron microscopy, the molecules involved in this process have not been identified, with the exception of the sorting receptor receptor-like membrane Ring-H2 (RMR; Jiang et al., 2000; Park et al., 2005, 2007; Hinz et al., 2007). In addition to this type of sorting mechanism in the Golgi stacks, the presence of an additional trafficking pathway has been demonstrated using the Arabidopsis (Arabidopsis thaliana) mutant atvsr1, which has a mutation in AtVSR1, a gene known to be involved in the sorting of lytic vacuolar proteins at the TGN (Shimada et al., 2003; Craddock et al., 2008; Fig. 1B). In this mutant, a relatively small proportion of PSV proteins was secreted into the apoplastic space, although a majority of PSV proteins were still normally targeted to the PSV. Thus, this route may constitute a salvage pathway for storage proteins that escape the aggregation sorting process in the cis-Golgi. In this mutant, lytic vacuolar proteins were also secreted in the seeds (Craddock et al., 2008), which concurs with the role of AtVSR1 in the sorting of lytic vacuolar proteins. Currently, it is not clear whether AtVSR1 plays a role in this salvage pathway directly or indirectly. In the atvsr1 mutant, a PSV-targeted chimeric reporter protein that did not contain any VSR-binding sequence was secreted into the apoplast, which led to the proposal that the absence of AtVSR1 indirectly affects the salvage pathway: the absence of AtVSR1 would affect the recycling of sorting effectors from the PVC and would thereby result in a pleiotropic alteration in membrane homeostasis, which in turn would cause missorting of PSV proteins (Craddock et al., 2008). Another possibility is that AtVSR1 plays a direct role in the sorting of storage proteins at the TGN. Arabidopsis 12S globulin, which was secreted in atvsr1, contains a specific sequence motif for the binding to AtVSR1 (Shimada et al., 2003). In addition, two PSV proteins, ricin and 2S albumin, of castor bean (Ricinus communis) contain the sequence-specific vacuolar sorting determinant (ssVSD) that binds to VSR/BP80-like proteins (Jolliffe et al., 2004). Thus, it is possible that both PSV and lytic vacuolar proteins reaching the TGN, either by escaping aggregation-mediated sorting at the cis-Golgi or by normal trafficking, are sorted by VSR/BP80 receptors at the TGN and transported to the PVC by the same pathway. Subsequently, these proteins may be subjected to additional sorting at the PVC, depending on their final destinations. However, this hypothesis will need to be tested in future studies. Another important question for protein targeting to the PSV is how membrane proteins are transported. Soluble proteins are transported to the PSV by the above two pathways; however, whether membrane proteins are also transported to the PSV through these two pathways remains unknown. There is a Golgi-independent pathway for the targeting of membrane proteins to the PSV, such as for α-tonoplast intrinsic protein (α-TIP; Jiang and Rogers, 1998; Park et al., 2004, 2007).
Proteins destined for the plasma membrane as well as secretory proteins are sorted at the TGN and then transported to their final destinations (Jurgens, 2004). In these cases, whether active sorting processes are required for these pathways is not clear, since proteins destined to the central lytic vacuole or the PSV are secreted out of the cell when vacuolar trafficking pathways are inhibited by various treatments, including overexpression of VSR/BP80 and RMR mutants or overexpression of the PVC-localized t-SNARE AtPEP12p (Shimada et al., 2003; Sohn et al., 2003; daSilva et al., 2005; Park et al., 2005; Foresti et al., 2006; Fuji et al., 2007; Craddock et al., 2008). The number of different routes for protein secretion and trafficking of plasma membrane proteins in plant cells is unknown. Besides protein cargoes, cell wall components such as matrix polysaccharides that are synthesized in the Golgi apparatus are packaged into secretory vesicles at the TGN and secreted into the extracellular space (for details, see review by Lerouxel et al., 2006). However, whether these cell wall components are secreted together with, or separately from, secretory proteins is unknown. Thus, these sorting processes are not clearly understood at the molecular level.
Another intriguing role of the TGN in plant cells is that it functions as the early endosome, an organelle that is involved in endocytosis (for details, see review by Lam et al., 2007). This conclusion is based on multiple lines of evidence, including the fact that the fluorescent dye FM4-64 is transported to the TGN immediately after internalization from the plasma membrane. In contrast, in animal cells and yeast, the early endosome is clearly separate from the TGN.
Finally, anterograde trafficking is balanced by retrograde trafficking originating from various post-Golgi compartments and moving to the Golgi apparatus (for details, see review by Jurgens, 2004). This retrograde trafficking plays a role in returning sorting receptors and other accessory proteins as well as lipid components of membranes. However, retrograde trafficking has been much more difficult to address experimentally compared with anterograde trafficking. Accordingly, progress in understanding these pathways has lagged behind anterograde trafficking pathways.
THE SORTING MOTIFS IDENTIFIED IN VACUOLAR PROTEINS IN PLANT PROTEINS
In plant cells, sorting motifs of soluble lytic vacuolar proteins at the Golgi apparatus have been studied extensively. Aleurain, a Cys protease found in aleurone in barley (Hordeum vulgare), and sporamin, a tuber storage protein in sweet potato (Ipomoea batatas), have been widely used as model proteins to study trafficking to the central lytic vacuole (Holwerda et al., 1990; Matsuoka and Nakamura, 1991). Targeting experiments employing various mutants of these proteins have revealed that the N-terminal propeptide region surrounding the amino acid sequence NPIR is essential for targeting to the central vacuole and can function as an independent motif. This region is called the ssVSD (Holwerda et al., 1992; Koide et al., 1999). The NPIR motif, together with the leader sequence of aleurain or sporamin, has been used to generate visible reporter proteins for vacuolar trafficking by constructing an N-terminal fusion to GFP or red fluorescent protein (Di Sansebastiano et al., 2001; Jin et al., 2001; Kim et al., 2001; Sohn et al., 2003; daSilva et al., 2005; Sanmartín et al., 2007). The amino acid sequence of the NPIR-type ssVSD differs from those of soluble lysosomal/vacuolar proteins in animal cells and yeast (Sly and Fisher, 1982; Jung et al., 1999). In animal cells, Man-6-P added to N-glycans is widely used as a lysosomal sorting signal by soluble hydrolases (Sly and Fisher, 1982).
In addition to the NPIR-type ssVSD, other motifs can direct proteins to the central vacuole. These motifs include the C-terminal propeptide (CTPP), which functions as a PSV sorting signal for several storage proteins localized to the PSV (Neuhaus and Rogers, 1998). However, CTPPs from chitinase A, concanavalin A, and amaranth (Amaranthus hypochondriacus) 11S globulin can redirect a secreted protein to the central vacuole in heterologous cells, such as in Arabidopsis, or in tobacco (Nicotiana tabacum) leaf cells or BY-2 cells, when fused C-terminally to a secreted protein (Di Sansebastiano et al., 1998; Claude et al., 2005; Hunter et al., 2007; Petruccelli et al., 2007; Sanmartín et al., 2007). However, whether these CTPPs represent bona fide sorting signals for the central vacuole is not clear.
The saposin-like plant-specific insert present in barley and soybean (Glycine max) aspartic proteinases may also function as a vacuolar sorting signal (Tormakangas et al., 2001; Simões and Faro, 2004). Additional evidence is needed to confirm the role of the saposin-like plant-specific insert in vacuolar sorting at the Golgi complex. In animal cells, the saposin-like domain plays a critical role in the targeting of acyloxyacyl hydrolase to lysosomes in the Man-6-P receptor (MPR)-independent pathway (Staab et al., 1994). In the berberine bridge enzyme in opium poppy (Papaver somniferum), a sequence motif responsible for central vacuolar targeting is located at amino acid positions 26 to 50 (Bird and Facchini, 2001). In addition, there are many soluble vacuolar proteins that do not contain any known sorting motifs but that may contain novel motifs.
In contrast to soluble proteins, the sorting signals of membrane proteins are largely unknown. Proteomics approaches have been used to identify a large number of vacuolar membrane proteins (Carter et al., 2004; Jaquinod et al., 2007). Of these, GFP fusion proteins of γ-TIP, a Ca2+-ATPase, and the Arabidopsis iron transporter AtNRAMP3 are targeted to the tonoplast of the central vacuole (Thomine et al., 2003; Hunter et al., 2007; Lee et al., 2007b). In animal cells, multiple sorting motifs have been identified in transmembrane proteins. These include the Tyr-based YXXΦ (where X is any amino acid and Φ is a hydrophobic amino acid), the dileucine [D/E]XXXL[L/I/M], and the acidic dileucine DXXLL motifs (Bonifacino, 2004; Traub, 2005). Additionally, monoubiquitin functions as a signal for membrane proteins to sort to the lysosome (Bonifacino and Traub, 2003; Piper and Luzio, 2007). The PVC-localized VSRs, which are single transmembrane domain (TMD) proteins, contain the YXXΦ motif at their C-terminal cytoplasmic domain (Carter et al., 2004; Happel et al., 2004). Mutation of Y to A in the YXXΦ motif of AtVSR2 (BP80a) causes mistargeting to the plasma membrane (daSilva et al., 2006), consistent with the hypothesis that the YXXΦ motif functions as a sorting motif. However, the sorting motifs for multi-TMD proteins remain entirely unknown.
The sorting motifs of PSV proteins have been identified. Three different types of sorting motifs have been identified: the CTPP, the internal physical and structural vacuolar sorting determinants (psVSDs), and the ssVSD (Neuhaus and Rogers, 1998; Maruyama et al., 2006). The CTPP has been identified in a lectin from barley, in chitinase, glucanase, and osmotin from tobacco, in 2S albumins from Brazil nut (Bertholletia excelsa) and pea (Pisum sativum), in phaseolin from the common bean (Phaseolus vulgaris), and in the β-conglycinin α′-subunit and 11S globulin of soybean (Neuhaus et al., 1991; Saalbach et al., 1991; Sticher et al., 1992; Dombrowski et al., 1993; Melchers et al., 1993; Frigerio et al., 1998; Neuhaus and Rogers, 1998; Nishizawa et al., 2003; Maruyama et al., 2006). In addition, a short sequence in the C-terminal cytosolic tail of α-TIP that targets to the PSV has been identified (Oufattole et al., 2005). Deletion of the CTPP from precursors causes secretion of the proteins, whereas fusion of the CTPPs from tobacco chitinase A, barley lectin, and soybean 11S globulin or β-conglycinin α′-subunit to reporter proteins targets the proteins to the PSV in soybean seed cells and seed cells of Arabidopsis transgenic plants (Nishizawa et al., 2003; Maruyama et al., 2006). These observations led to the conclusion that the CTPP is necessary and sufficient for correct targeting to the vacuole. However, the CTPP does not reveal any clear consensus sequence, with the exception that it is rich in hydrophobic amino acids (Neuhaus et al., 1994; Robinson et al., 2005). Thus, the exact molecular nature of the CTPP is not clearly understood at present.
The internal psVSD is not as thoroughly characterized, but two different subgroups have been identified, based on their biochemical properties. One type of psVSD may be composed of multiple internal domains that form a higher order structure to function as a sorting determinant, as observed with legumin, in which multiple domains appear to act as vacuolar sorting signals (Saalbach et al., 1991). The other type of psVSD is formed by the aggregation of proteins (Vitale and Chrispeels, 1992). Some storage proteins form high molecular mass protein aggregates (Hinz et al., 1997; Neuhaus and Rogers, 1998; Castelli and Vitale, 2005), and protein aggregation may occur in the Golgi apparatus (Hillmer et al., 2001; Hinz et al., 2007). Proproteins are often more hydrophobic than their mature counterparts and have a tendency to form aggregates (Hinz et al., 1997; Castelli and Vitale, 2005).
In addition to these CTPP and psVSD sorting signals for PSV proteins, an ssVSD-type sorting motif has been identified in ricin and the 2S albumin of castor bean and in glycinin group I subunit A1aB1b and group II subunit A3B4 of soybean (Frigerio et al., 2001; Brown et al., 2003; Maruyama et al., 2006). However, in the case of the glycinin group I protein A1aB1b, it is not clear whether this ssVSD functions as a PSV sorting motif in vivo because this protein also contains the typical CTPP. In contrast, in the case of the group II subunit A3B4 of soybean glycinin, this protein contains only an ssVSD, and not a CTPP, indicating that the ssVSD must be functional in targeting the protein to the PSV (Maruyama et al., 2006). In addition, the C-terminal three-amino acid motif contained in the CTPP of soybean β-conglycinin α′-subunit can act as an ssVSD at the N terminus (Nishizawa et al., 2003).
The large number of sorting motifs that have been identified in a wide variety of proteins leads to questions about the nature of the sorting receptors that recognize these sorting motifs. Among the three types of sorting motifs found in soluble proteins, lytic vacuolar proteins preferentially use the ssVSD, whereas PSV proteins use the sorting motifs CTPP and psVSD. This difference in the use of sorting motifs may stem from differences in the sorting mechanisms and/or sorting receptors. One possibility is that VSRs may recognize multiple ssVSD-type sorting motifs in a variety of lytic vacuolar proteins despite differences in the amino acid sequences of ssVSDs (Ahmed et al., 2000; Jolliffe et al., 2004). Similarly, RMR may recognize multiple CTPP-type sorting motifs in various PSV proteins (Park et al., 2005, 2007). In addition, sequence-specific sorting motifs may be recognized by specialized sorting receptors, as observed for the protein-specific sorting receptor LIMP-II in animal cells (Reczek et al., 2007).
THE RECEPTOR FOR PROTEIN SORTING AT THE GOLGI APPARATUS
The search for vacuolar sorting receptors led to the identification of an 80-kD protein called BP80 (Kirsch et al., 1994, 1996). A member of the highly conserved VSR family of plant proteins (Kirsch et al., 1996; Ahmed et al., 1997; Paris et al., 1997; Cao et al., 2000; Paris and Neuhaus, 2002), BP80 was originally identified from lysates of pea CCV membranes, where it acted as a proaleurain ssVSD-binding protein. BP80 binds specifically to the NPIR motif of the ssVSDs of the soluble vacuolar proteins sporamin and aleurain (Fig. 1A). The Arabidopsis homolog of BP80, AtELP (identical to AtVSR1), which displays homology to the epidermal growth factor receptor in animals, was identified independently as a protein that binds to ssVSD-containing vacuolar proteins (Ahmed et al., 2000). AtELP/AtVSR1, a member of the VSR family, is a type I transmembrane protein with a large N-terminal luminal domain, a single TMD, and a short C-terminal cytoplasmic domain (CCD). These features are characteristic of sorting receptors at the TGN, such as MPRs and Vps10 in animal cells and yeast, respectively (Dintzis et al., 1994; Honing et al., 1997; Seaman et al., 1997). The large luminal domain contains two regions of homology: a protease-associated domain and an epidermal growth factor receptor-like domain (Cao et al., 2000). Multiple isoforms of VSRs exist in various plant species; for example, Arabidopsis contains seven isoforms (Hadlington and Denecke, 2000). Phylogenetic analysis of the CCD revealed that these multiple VSR isoforms can be divided into three distinct subgroups (Masclaux et al., 2005), although the functional significance of this grouping is unclear.
Paris et al. (1997) demonstrated that BP80 was present on the Golgi apparatus and small vacuolar structures, consistent with the appearance of PVC. Subsequently, it has been confirmed that VSRs localize primarily to the PVC in Arabidopsis and BY-2 cells (Sanderfoot et al., 1998; Li et al., 2002; Tse et al., 2004; Miao et al., 2006; daSilva et al., 2006), and the TMD and CCD are sufficient for this localization. In addition, a minor fraction of VSR proteins are localized to the TGN (Sanderfoot et al., 1998; Hinz et al., 2007). The TGN-localized proteins represent VSRs that have been returned from the PVC to the TGN for binding to soluble vacuolar proteins via the NPIR-type sorting motif. Consistent with this hypothesis, daSilva et al. (2005) have shown that VSR salvage from the PVC to the TGN is critical for the vacuolar trafficking of soluble cargo proteins. At the TGN, VSR/cargo complexes are packaged into clathrin-coated buds, which are subsequently released as CCVs. Soon after release from the TGN, CCVs may be uncoated and fuse with the PVC, where the cargo proteins are released from the VSR proteins (Neuhaus and Rogers, 1998; Jurgens, 2004; Masclaux et al., 2005; Robinson et al., 2005). The lower pH in the PVC may play a role in the release of cargo proteins from VSRs. The role of the PVC in protein trafficking in plant cells was the subject of a recent review (Mo et al., 2006).
The functional significance of VSR/BP80 in vacuolar trafficking has been demonstrated. In protoplasts, overexpression of a VSR/BP80 mutant, in which the luminal domain was replaced with GFP, caused secretion of a soluble vacuolar protein (daSilva et al., 2005). However, a loss-of-function mutation in AtVSR1 does not display any noticeable phenotype in lytic vacuolar trafficking, possibly due to the presence of multiple VSR isoforms in Arabidopsis. In contrast, the atvsr1 mutant in seed cells secretes its storage proteins, such as 12S globulin, to the apoplast (Shimada et al., 2003). In addition, the large number of vacuolar proteins raises the possibility that additional vacuolar sorting receptors may also exist in plant cells (Carter et al., 2004). However, other vacuolar sorting receptors have not been identified in plant cells. In fact, multiple vacuolar sorting receptors, such as sortilin and MPR-like proteins, are found in animal cells and yeast, respectively (Whyte and Munro, 2001; Ni et al., 2006). In addition, the protein-specific sorting receptor LIMP-II has been identified in animal cells (Reczek et al., 2007).
The sorting receptors for PSV proteins are less well characterized. In Arabidopsis, RMR is a sorting receptor primarily localized to the PVC (Jiang et al., 2000; Park et al., 2005, 2007; Hinz et al., 2007), with minor fractions in the Golgi apparatus and the PSV. Interestingly, RMR interacts with the C-terminal sorting motif of phaseolin and chitinase aggregates, suggesting that aggregate formation of PSV proteins in the Golgi stacks is a prerequisite for the interaction between the sorting receptor and cargo proteins (Park et al., 2005, 2007; Fig. 1B). In fact, this is consistent with the results showing that PSV proteins form aggregates at the Golgi apparatus starting at the cis-Golgi (Hillmer et al., 2001; Hinz et al., 2007). In addition, as mentioned earlier, VSRs play a role as a sorting receptor for PSV proteins (Shimada et al., 2003; Fuji et al., 2007; Fig. 1B). In this process, VSRs bind to a specific sorting motif present in Arabidopsis 12S globulin and in ricin and 2S albumin of castor bean, thereby acting as a sorting receptor for these proteins at the TGN (Shimada et al., 2003; Jolliffe et al., 2004).
ADAPTOR PROTEINS INVOLVED IN TRAFFICKING FROM THE GOLGI APPARATUS TO THE POST-GOLGI COMPARTMENTS
The VSRs bound to cargo proteins at the TGN must be packaged into CCVs. However, the detailed mechanism by which this occurs remains poorly understood. Since the biological role of VSRs is most similar to that of yeast Vps10 and animal MPR (Dintzis et al., 1994; Honing et al., 1997; Seaman et al., 1997), the mechanisms of action of these proteins may also be similar. In Vps10p and MPRs, the CCD contains motifs for interaction with adaptors such as AP-1 and Golgi-localized γ-ear containing Arf binding (GGA; Nakatsu and Ohno, 2003). The interaction between adaptors and sorting receptors is essential for trafficking to the lysosome/vacuole. Similarly, the CCD of VSRs contains the YXXΦ motif. In VSR/BP80, the YXXΦ motif of VSR-PS1 in pea binds in vitro to Arabidopsis μA, a homolog of the μ-adaptin of AP in animal cells (Happel et al., 2004). Accordingly, VSR/BP80 in Arabidopsis may interact with AP-1 as an adaptor protein. Consistent with this hypothesis, the Arabidopsis genome encodes proteins homologous to components of all four AP complexes (Robinson, 2004). In addition, Song et al. (2006) demonstrated that AtVSR1 interacts with Arabidopsis EpsinR1 (formally EPSIN1), a homolog of animal EpsinR/clint that functions as an adaptor in CCV-mediated lysosomal trafficking of soluble cargo proteins (Kalthoff et al., 2002). EpsinR1 also interacts with clathrin, actin filaments, and AP-1 (Song et al., 2006), indicating that AP-1 and/or EpsinR1 function as adaptors for the packaging of AtVSR1/cargo complexes into CCVs. Actin filaments may play a role in recruiting EpsinR1 to the TGN, which facilitates vesicle formation (Song et al., 2006). Consistent with this idea, disassembly of actin filaments by latrunculin B strongly inhibited vacuolar trafficking of soluble proteins and resulted in their accumulation in the Golgi apparatus (Kim et al., 2005).
Animal cells and yeast contain another type of adaptor protein, the monomeric GGA adaptors (Bonifacino, 2004). In the Arabidopsis genome, genes such as At3g08790 and At4g32760 encode proteins with VHS (Vps27, Hrs, and STAM) and GAT (GGA and TOM) domains. However, they display a low degree of amino acid sequence similarity to animal GGAs. Thus, it is unclear whether these plant proteins are homologs of GGAs and function as adaptors for vacuolar trafficking at the Golgi complex.
Plant cells also contain a protein that is homologous to δ-adaptin of AP-3 (Lee et al., 2007a; Fig. 1A). Lee et al. (2007a) demonstrated that Arabidopsis δ-adaptin interacts with EpsinR2, which binds to a v-SNARE AtVTI12, clathrin, and phospholipid phosphatidylinositol 3-phosphate. These observations imply that plant cells may also transport proteins to the central lytic vacuole by a route that differs from that used by BP80/VSR for soluble vacuolar proteins with the NPIR sorting motif. Consistent with this hypothesis, δ-adaptin localizes to an undefined, non-PVC organelle. This observation supports the idea that AP-3 and EpsinR2 may together mediate the trafficking of proteins to the vacuole. Interestingly, terminal flower 1 (TFL1), which has been suggested to play a role in PSV trafficking, localizes to a δ-adaptin-positive compartment (Sohn et al., 2007). In addition, Sanmartín et al. (2007) suggested that AtVTI12 plays a role in PSV trafficking. Thus, additional studies are necessary to clarify whether AP-3 and/or EpsinR2 play a role in both lytic vacuolar trafficking and PSV trafficking.
OTHER PROTEINS INVOLVED IN ANTEROGRADE TRAFFICKING FROM THE GOLGI APPARATUS
In addition to these adaptor proteins and their interacting proteins, many proteins implicated in anterograde trafficking from the TGN have been identified and characterized at the molecular level. These include SNAREs such as AtVTI11, AtVTI12, SYP51, SYP61, AtSYP41, and AtSYP42 (Bassham and Raikhel, 1998; Zheng et al., 1999; Bassham et al., 2000; Sanderfoot et al., 2001). AtVTI11 is a v-SNARE that interacts with the t-SNARE AtPEP12p at the PVC (Sanderfoot et al., 1998; Zheng et al., 1999). AtVTI11 also interacts with the monomeric adaptin EpsinR1 (Song et al., 2006), implying that VTI11 is recruited to the TGN by EpsinR1 as a v-SNARE during the formation of CCVs. Interestingly, the Arabidopsis mutant atvti11 displays a defect in gravitropism (Kato et al., 2002). However, it is not clearly understood how the loss of AtVTI11 leads to the defect in gravitropism. As mentioned above, AtVTI12, a close homolog of AtVTI11, is thought to play a role in the PSV pathway (Sanmartín et al., 2007). The Arabidopsis mutant atvti12 displays an accelerated senescence phenotype in poor media (Surpin et al., 2003).
ADL6/DRP2A (for Arabidopsis dynamin-like protein 6/dynamin-related protein 2A) and its interacting proteins have been shown to play a role in the TGN for the central lytic vacuolar trafficking pathways. Dynamin, a large GTPase, functions in severing the neck of the coated bud from the plasma membrane as a vesicle during endocytosis (Ungewickell and Hinrichsen, 2007). At the TGN, ADL6/DRP2A may play a role in releasing the CCV from the TGN (Jin et al., 2001; Lam et al., 2002). An Arabidopsis SH3 domain-containing protein, AtSH3P3, interacts with ADL6/DRP2A, and these proteins may coordinately mediate this process (Lam et al., 2001). Thus, complex networks of protein-protein interactions among these proteins may be critical for efficient protein trafficking.
The TGN-localized small GTPase-related proteins, including Arf1 and Arf1-related proteins, as well as Rabs and Rab-related proteins, also play critical roles in anterograde trafficking. Originally, Arf1 was shown to play a role in the recruitment of COPI components to the Golgi apparatus for retrograde trafficking from the Golgi apparatus to the ER (Roth, 1999; Lee et al., 2002; Takeuchi et al., 2002; Memon, 2004). In addition, Arf1 has been shown to play a role in vacuolar trafficking (Pimpl et al., 2003), raising the possibility that the Arf1 and Arf1-related plant proteins may play a role in vacuolar trafficking at the TGN. In animal cells, Arf1 plays an essential role by binding to and recruiting monomeric adaptor GGAs to the TGN (Shiba et al., 2003). However, the exact role of Arf1 in anterograde trafficking at the TGN in plant cells is not clear. Rabs regulate the four major steps in membrane traffic: vesicle budding, vesicle delivery, vesicle tethering, and fusion of the vesicle membrane with that of the target compartment (Grosshans et al., 2006). The Rab proteins involved in anterograde trafficking at the TGN are the subject of a recent review (Hanton et al., 2007). RabE1d is involved in trafficking from the TGN to the plasma membrane (Zheng et al., 2005), whereas m-Rabmc, an N-myristoylated Rab-GTPase in Mesembryanthemum crystallinum, is involved in vacuolar trafficking from the Golgi apparatus (Bolte et al., 2004). RabA4b is involved in polarized secretion from the TGN in growing root hair cells (Preuss et al., 2004), and Rab-A2 and Rab-A3 contribute substantially to cell plate formation (Chow et al., 2008). However, it is not fully understood how these Rab proteins contribute to root hair development or cell plate formation. They may regulate protein trafficking in various pathways that ultimately play a role in these processes. In addition to Rab proteins, a Rab GTPase-activating protein (GAP) plays a role in plant cell protein trafficking. Rice (Oryza sativa) GAP1, which stimulates the activity of the rice Rabs OsRab8a and OsRab11 in vitro, is involved in anterograde trafficking from the TGN to the plasma membrane and the PVC (Heo et al., 2005).
Another class of proteins that play important roles at the TGN with respect to protein trafficking includes the TGN-localized V-ATPase isoform VHA-a1 and the chloride transporter AtCLC-d, which control the luminal pH of the TGN (Dettmer et al., 2006; von der Fecht-Bartenbach et al., 2007; Brüx et al., 2008). The pH may be critical for interactions between sorting receptors and cargo proteins in the lumen of the TGN. Similarly, in animal cells, the pH of the TGN is important for the processing, sorting, and transport of secreted cargo proteins at the TGN (Demaurex et al., 1998).
Another important player that influences protein trafficking from the TGN to the vacuole is the retromer. VPS29 and VPS35, two of the five retromer components, are necessary for efficient targeting of PSV proteins in seeds, which suggests that the retromer may play a role in the recycling of cargo receptors, likely VSRs, to the TGN (Shimada et al., 2006; Yamazaki et al., 2008). In addition, a VSR is coimmunoprecipitated with VPS35 by an anti-VPS35 antibody, implying that the retromer is involved in recycling of the BP80/VSRs from the PVC to the TGN (Oliviusson et al., 2006).
PERSPECTIVES
Recently, a large number of proteins involved in protein trafficking were identified and characterized at the molecular level, which has been instrumental for depicting the molecular mechanisms of protein trafficking at various steps in trafficking in plant cells. However, the mechanisms of action of these newly identified molecules are not fully understood. For example, we still lack an understanding of the trafficking of most membrane proteins to the tonoplast of the central vacuole and to the plasma membrane as well as of the secretion of cell wall materials. In certain cases, recent studies have demonstrated the presence of trafficking routes from and to the Golgi apparatus with very few factors involved in these pathways. In other cases, such as proteins with Epsin/ap180 N-terminal homology domains, the plant cell proteins have homology to proteins found in animal cells and yeast, but the precise role of the protein in plants has not yet been confirmed (Holstein and Oliviusson, 2005). Thus, the molecular players identified to date may constitute only a fraction of the proteins that function in sorting and anterograde trafficking from the Golgi apparatus to the post-Golgi compartments in plant cells. In the future, a large number of additional factors may be identified and characterized at the molecular and cellular levels, which will be used in future studies to elucidate the detailed mechanism(s) of protein trafficking at the molecular and cellular levels.
Another exciting development is the discovery that protein trafficking is intimately linked to many different biological processes, such as plant development, biotic and abiotic responses, and hormone-mediated signaling. During plant development, the physiological conditions of plant cells may require changes in protein trafficking, which may produce cell type-specific conditions in trafficking. In addition, protein trafficking is modulated in response to environmental challenges such as infection by pathogens and abiotic stresses. In fact, the overall rate of protein trafficking has been shown to change in response to pathogen infection (Hardham et al., 2008). This linkage is due to the fact that the proper distribution, both spatially and temporally, of proteins to various organelles by protein trafficking is essential to other biological processes (Geldner, 2004; Surpin and Raikhel, 2004; Leshem et al., 2006; Robatzek, 2007). Thus, an exciting outcome of current research will be our understanding of how protein trafficking is connected to these various aspects of plant biology at the cell and molecular levels.
This work was supported by grants from the Creative Research Program of the Ministry of Education, Science and Technology, by Biogreen21 (grant no. 20070401–034–026–008–03–001) of the Rural Development Administration and Agricultural Research and Planning Center, Ministry of Agriculture, Forestry and Foods, and by the Core Research Fund of Pohang University of Science and Technology, Korea.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Inhwan Hwang (ihhwang@postech.ac.kr).
References
- Ahmed SU, Bar-Peled M, Raikhel NV (1997) Cloning and subcellular location of an Arabidopsis receptor-like protein that shares common features with protein-sorting receptors of eukaryotic cells. Plant Physiol 114 325–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed SU, Rojo E, Kovaleva V, Venkataraman S, Dombrowski JE, Matsuoka K, Raikhel NV (2000) The plant vacuolar sorting receptor AtELP is involved in transport of NH(2)-terminal propeptide-containing vacuolar proteins in Arabidopsis thaliana. J Cell Biol 149 1335–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassham DC, Raikhel NV (1998) An Arabidopsis VPS45p homolog implicated in protein transport to the vacuole. Plant Physiol 117 407–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassham DC, Sanderfoot AA, Kovaleva V, Zheng H, Raikhel NV (2000) AtVPS45 complex formation at the trans-Golgi network. Mol Biol Cell 11 2251–2265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baust T, Anitei M, Czupalla C, Parshyna I, Bourel L, Thiele C, Krause E, Hoflack B (2008) Protein networks supporting AP-3 function in targeting lysosomal membrane proteins. Mol Biol Cell 19 1942–1951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird DA, Facchini PJ (2001) Berberine bridge enzyme, a key branch-point enzyme in benzylisoquinoline alkaloid biosynthesis, contains a vacuolar sorting determinant. Planta 213 888–897 [DOI] [PubMed] [Google Scholar]
- Bolte S, Brown S, Satiat-Jeunemaitre B (2004) The N-myristoylated Rab-GTPase m-Rabmc is involved in post-Golgi trafficking events to the lytic vacuole in plant cells. J Cell Sci 117 943–954 [DOI] [PubMed] [Google Scholar]
- Bonifacino JS (2004) The GGA proteins: adaptors on the move. Nat Rev Mol Cell Biol 5 23–32 [DOI] [PubMed] [Google Scholar]
- Bonifacino JS, Traub LM (2003) Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu Rev Biochem 72 395–447 [DOI] [PubMed] [Google Scholar]
- Bowers K, Stevens TH (2005) Protein transport from the late Golgi to the vacuole in the yeast Saccharomyces cerevisiae. Biochim Biophys Acta 1744 438–454 [DOI] [PubMed] [Google Scholar]
- Brown JC, Jolliffe NA, Frigerio L, Roberts LM (2003) Sequence-specific, Golgi-dependent vacuolar targeting of castor bean 2S albumin. Plant J 36 711–719 [DOI] [PubMed] [Google Scholar]
- Brüx A, Liu TY, Krebs M, Stierhof YD, Lohmann JU, Miersch O, Wasternack C, Schumacher K (2008) Reduced V-ATPase activity in the trans-Golgi network causes oxylipin-dependent hypocotyl growth inhibition in Arabidopsis. Plant Cell 20 1088–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Rogers SW, Butle J, Beevers L, Rogers JC (2000) Structural requirements for ligand binding by a probable plant vacuolar sorting receptor. Plant Cell 12 493–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter C, Pan S, Zouhar J, Avila EL, Girke T, Raikhel NV (2004) The vegetative vacuole proteome of Arabidopsis thaliana reveals predicted and unexpected proteins. Plant Cell 16 3285–3303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelli S, Vitale A (2005) The phaseolin vacuolar sorting signal promotes transient, strong membrane association and aggregation of the bean storage protein in transgenic tobacco. J Exp Bot 56 1379–1387 [DOI] [PubMed] [Google Scholar]
- Chow CM, Neto H, Foucart C, Moore I (2008) Rab-A2 and Rab-A3 GTPases define a trans-Golgi endosomal membrane domain in Arabidopsis that contributes substantially to the cell plate. Plant Cell 20 101–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claude SJ, Marie-Agnès G, Catalina R, Nadine P, Marie-Christine KM, Jean-Marc N, Loïc F, Véronique G (2005) Targeting of proConA to the plant vacuole depends on its nine amino-acid C-terminal propeptide. Plant Cell Physiol 46 1603–1612 [DOI] [PubMed] [Google Scholar]
- Craddock CP, Hunter PR, Szakacs E, Hinz G, Robinson DG, Frigerio L (2008) Lack of a vacuolar sorting receptor leads to non-specific missorting of soluble vacuolar proteins in Arabidopsis seeds. Traffic 9 408–416 [DOI] [PubMed] [Google Scholar]
- Crowley KS, Liao S, Worrell VE, Reinhart GD, Johnson AE (1994) Secretory proteins move through the endoplasmic reticulum membrane via an aqueous, gated pore. Cell 78 461–471 [DOI] [PubMed] [Google Scholar]
- daSilva LL, Foresti O, Denecke J (2006) Targeting of the plant vacuolar sorting receptor BP80 is dependent on multiple sorting signals in the cytosolic tail. Plant Cell 18 1477–1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- daSilva LL, Taylor JP, Hadlington JL, Hanton SL, Snowden CJ, Fox SJ, Foresti O, Brandizzi F, Denecke J (2005) Receptor salvage from the prevacuolar compartment is essential for efficient vacuolar protein targeting. Plant Cell 17 132–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaurex N, Furuya W, D'Souza S, Bonifacino JS, Grinstein S (1998) Mechanism of acidification of the trans-Golgi network (TGN): in situ measurements of pH using retrieval of TGN38 and furin from the cell surface. J Biol Chem 273 2044–2051 [DOI] [PubMed] [Google Scholar]
- Dettmer J, Hong-Hermesdorf A, Stierhof YD, Schumacher K (2006) Vacuolar H+-ATPase activity is required for endocytic and secretory trafficking in Arabidopsis. Plant Cell 18 715–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dintzis SM, Velculescu VE, Pfeffer SR (1994) Receptor extracellular domains may contain trafficking information: studies of the 300-kDa mannose 6-phosphate receptor. J Biol Chem 269 12159–12166 [PubMed] [Google Scholar]
- Di Sansebastiano GP, Paris N, Marc-Martin S, Neuhaus JM (1998) Specific accumulation of GFP in a non-acidic vacuolar compartment via a C-terminal propeptide-mediated sorting pathway. Plant J 15 449–457 [DOI] [PubMed] [Google Scholar]
- Di Sansebastiano GP, Paris N, Marc-Martin S, Neuhaus JM (2001) Regeneration of a lytic central vacuole and of neutral peripheral vacuoles can be visualized by green fluorescent proteins targeted to either type of vacuoles. Plant Physiol 126 78–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombrowski JE, Schroeder MR, Bednarek SY, Raikhel NV (1993) Determination of the functional elements within the vacuolar targeting signal of barley lectin. Plant Cell 5 587–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foresti O, daSilva LL, Denecke J (2006) Overexpression of the Arabidopsis syntaxin PEP12/SYP21 inhibits transport from the prevacuolar compartment to the lytic vacuole in vivo. Plant Cell 18 2275–2293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigerio L, de Virgilio M, Prada A, Faoro F, Vitale A (1998) Sorting of phaseolin to the vacuole is saturable and requires a short C-terminal peptide. Plant Cell 10 1031–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigerio L, Jolliffe NA, Di Cola A, Felipe DH, Paris N, Neuhaus JM, Lord JM, Ceriotti A, Roberts LM (2001) The internal propeptide of the ricin precursor carries a sequence-specific determinant for vacuolar sorting. Plant Physiol 126 167–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuji K, Shimada T, Takahashi H, Tamura K, Koumoto Y, Utsumi S, Nishizawa K, Maruyama N, Hara-Nishimura I (2007) Arabidopsis vacuolar sorting mutants (green fluorescent seed) can be identified efficiently by secretion of vacuole-targeted green fluorescent protein in their seeds. Plant Cell 19 597–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geldner N (2004) The plant endosomal system: its structure and role in signal transduction and plant development. Planta 219 547–560 [DOI] [PubMed] [Google Scholar]
- Grosshans BL, Ortiz D, Novick P (2006) Rabs and their effectors: achieving specificity in membrane traffic. Proc Natl Acad Sci USA 103 11821–11827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu F, Crump CM, Thomas G (2001) Trans-Golgi network sorting. Cell Mol Life Sci 58 1067–1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadlington JL, Denecke J (2000) Sorting of soluble proteins in the secretory pathway of plants. Curr Opin Plant Biol 3 461–468 [DOI] [PubMed] [Google Scholar]
- Hanton SL, Matheson LA, Chatre L, Rossi M, Brandizzi F (2007) Post-Golgi protein traffic in the plant secretory pathway. Plant Cell Rep 26 1431–1438 [DOI] [PubMed] [Google Scholar]
- Happel N, Honing S, Neuhaus JM, Paris N, Robinson DG, Holstein SE (2004) Arabidopsis mu A-adaptin interacts with the tyrosine motif of the vacuolar sorting receptor VSR-PS1. Plant J 37 678–693 [DOI] [PubMed] [Google Scholar]
- Hardham AR, Takemoto D, White RG (2008) Rapid and dynamic subcellular reorganization following mechanical stimulation of Arabidopsis epidermal cells mimics responses to fungal and oomycete attack. BMC Plant Biol 8 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo JB, Rho HS, Kim SW, Hwang SM, Kwon HJ, Nahm MY, Bang WY, Bahk JD (2005) OsGAP1 functions as a positive regulator of OsRab11-mediated TGN to PM or vacuole trafficking. Plant Cell Physiol 46 2005–2018 [DOI] [PubMed] [Google Scholar]
- Hillmer S, Movafeghi A, Robinson DG, Hinz G (2001) Vacuolar storage proteins are sorted in the cis-cisternae of the pea cotyledon Golgi apparatus. J Cell Biol 152 41–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz G, Colanesi S, Hillmer S, Rogers JC, Robinson DG (2007) Localization of vacuolar transport receptors and cargo proteins in the Golgi apparatus of developing Arabidopsis embryos. Traffic 8 1452–1464 [DOI] [PubMed] [Google Scholar]
- Hinz G, Hillmer S, Bäumer M, Hohl I (1999) Vacuolar storage proteins and the putative vacuolar sorting receptor BP-80 exit the Golgi apparatus of developing pea cotyledons in different transport vesicles. Plant Cell 11 1509–1524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz G, Menze A, Hohl I, Vaux D (1997) Isolation of prolegumin from developing pea seeds: its binding to endomembranes and assembly into prolegumin hexamers in the protein storage vacuole. J Exp Bot 48 139–149 [Google Scholar]
- Holstein SE, Oliviusson P (2005) Sequence analysis of Arabidopsis thaliana E/ANTH-domain-containing proteins: membrane tethers of the clathrin-dependent vesicle budding machinery. Protoplasma 226 13–21 [DOI] [PubMed] [Google Scholar]
- Holwerda BC, Galvin NJ, Baranski TJ, Rogers JC (1990) In vitro processing of aleurain, a barley vacuolar thiol protease. Plant Cell 2 1091–1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holwerda BC, Padgett HS, Rogers JC (1992) Proaleurain vacuolar targeting is mediated by short contiguous peptide interactions. Plant Cell 4 307–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honing S, Sandoval IV, von Figura K (1998) A di-leucine-based motif in the cytoplasmic tail of LIMP-II and tyrosinase mediates selective binding of AP-3. EMBO J 17 1304–1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honing S, Sosa M, Hille-Rehfeld A, von Figura K (1997) The 46-kDa mannose 6-phosphate receptor contains multiple binding sites for clathrin adaptor. J Biol Chem 272 19884–19890 [DOI] [PubMed] [Google Scholar]
- Hunter PR, Craddock CP, Di Benedetto S, Roberts LM, Frigerio L (2007) Fluorescent reporter proteins for the tonoplast and the vacuolar lumen identify a single vacuolar compartment in Arabidopsis cells. Plant Physiol 145 1371–1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaquinod M, Villiers F, Kieffer-Jaquinod S, Hugouvieux V, Bruley C, Garin J, Bourguignon J (2007) A proteomics dissection of Arabidopsis thaliana vacuoles isolated from cell culture. Mol Cell Proteomics 6 394–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Phillips TE, Rogers SW, Rogers JC (2000) Biogenesis of the protein storage vacuole crystalloid. J Cell Biol 150 755–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Rogers JC (1998) Integral membrane protein sorting to vacuoles in plant cells: evidence for two pathways. J Cell Biol 143 1183–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin JB, Kim YA, Kim SJ, Lee SH, Kim DH, Cheong GW, Hwang I (2001) A new dynamin-like protein, ADL6, is involved in trafficking from the trans-Golgi network to the central vacuole in Arabidopsis. Plant Cell 13 1511–1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolliffe NA, Brown JC, Neumann U, Vicré M, Bachi A, Hawes C, Ceriotti A, Roberts LM, Frigerio L (2004) Transport of ricin and 2S albumin precursors to the storage vacuoles of Ricinus communis endosperm involves the Golgi and VSR-like receptors. Plant J 39 821–833 [DOI] [PubMed] [Google Scholar]
- Jolliffe NA, Craddock CP, Frigerio L (2005) Pathways for protein transport to seed storage vacuoles. Biochem Soc Trans 33 1016–1018 [DOI] [PubMed] [Google Scholar]
- Jung G, Ueno H, Hayashi R (1999) Carboxypeptidase Y: structural basis for protein sorting and catalytic triad. J Biochem 126 1–6 [DOI] [PubMed] [Google Scholar]
- Jurgens G (2004) Membrane trafficking in plants. Annu Rev Cell Dev Biol 20 481–504 [DOI] [PubMed] [Google Scholar]
- Kalthoff C, Groos S, Kohl R, Mahrhold S, Ungewickell EJ (2002) Clint: a novel clathrin-binding ENTH-domain protein at the Golgi. Mol Biol Cell 13 4060–4073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T, Morita MT, Fukaki H, Yamauchi Y, Uehara M, Niihama M, Tasaka M (2002) SGR2, a phospholipase-like protein, and ZIG/SGR4, a SNARE, are involved in the shoot gravitropism of Arabidopsis. Plant Cell 14 33–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DH, Eu YJ, Yoo CM, Kim YW, Pih KT, Jin JB, Kim SJ, Stenmark H, Hwang I (2001) Trafficking of phosphatidylinositol 3-phosphate from the trans-Golgi network to the lumen of the central vacuole in plant cells. Plant Cell 13 287–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Park M, Kim SJ, Hwang I (2005) Actin filaments play a critical role in vacuolar trafficking at the Golgi complex in plant cells. Plant Cell 17 888–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch T, Paris N, Butler JM, Beevers L, Rogers JC (1994) Purification and initial characterization of a potential plant vacuolar targeting receptor. Proc Natl Acad Sci USA 91 3403–3407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch T, Saalbach G, Raikhel NV, Beevers L (1996) Interaction of a potential vacuolar targeting receptor with amino- and carboxyl-terminal targeting determinants. Plant Physiol 111 469–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koide Y, Matsuoka K, Ohto M, Nakamura K (1999) The N-terminal propeptide and the C terminus of the precursor to 20-kilo-dalton potato tuber protein can function as different types of vacuolar sorting signals. Plant Cell Physiol 40 1152–1159 [DOI] [PubMed] [Google Scholar]
- Lam BC, Sage TL, Bianchi F, Blumwald E (2001) Role of SH3 domain-containing proteins in clathrin-mediated vesicle trafficking in Arabidopsis. Plant Cell 11 2499–2512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam BC, Sage TL, Bianchi F, Blumwald E (2002) Regulation of ADL6 activity by its associated molecular network. Plant J 31 565–576 [DOI] [PubMed] [Google Scholar]
- Lam SK, Tse YC, Robinson DG, Jiang L (2007) Tracking down the elusive early endosome. Trends Plant Sci 12 497–505 [DOI] [PubMed] [Google Scholar]
- Lee GJ, Kim H, Kang H, Jang M, Lee DW, Lee S, Hwang I (2007. a) EpsinR2 interacts with clathrin, adaptor protein-3, AtVTI12, and phosphatidylinositol-3-phosphate: implications for EpsinR2 function in protein trafficking in plant cells. Plant Physiol 143 1561–1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MH, Min MK, Lee YJ, Jin JB, Shin DH, Kim DH, Lee KH, Hwang I (2002) ADP-ribosylation factor 1 of Arabidopsis plays a critical role in intracellular trafficking and maintenance of endoplasmic reticulum morphology in Arabidopsis. Plant Physiol 129 1507–1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SM, Kim HS, Han HJ, Moon BC, Kim CY, Harper JF, Chung WS (2007. b) Identification of a calmodulin-regulated autoinhibited Ca2+-ATPase (ACA11) that is localized to vacuole membranes in Arabidopsis. FEBS Lett 581 3943–3949 [DOI] [PubMed] [Google Scholar]
- Lerouxel O, Cavalier DM, Liepman AH, Keegstra K (2006) Biosynthesis of plant cell wall polysaccharides: a complex process. Curr Opin Plant Biol 9 621–630 [DOI] [PubMed] [Google Scholar]
- Leshem Y, Melamed-Book N, Cagnac O, Ronen G, Nishri Y, Solomon M, Cohen G, Levine A (2006) Suppression of Arabidopsis vesicle-SNARE expression inhibited fusion of H2O2-containing vesicles with tonoplast and increased salt tolerance. Proc Natl Acad Sci USA 103 18008–18013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YB, Rogers SW, Tse YC, Lo SW, Sun SS, Jauh GY, Jiang L (2002) BP-80 and homologs are concentrated on post-Golgi, probable lytic prevacuolar compartments. Plant Cell Physiol 43 726–742 [DOI] [PubMed] [Google Scholar]
- Marcusson EG, Horazdovsky BF, Cereghino JL, Gharakhanian E, Emr SD (1994) The sorting receptor for yeast vacuolar carboxypeptidase Y is encoded by the VPS10 gene. Cell 77 579–586 [DOI] [PubMed] [Google Scholar]
- Maruyama N, Mun LC, Tatsuhara M, Sawada M, Ishimoto M, Utsumi S (2006) Multiple vacuolar sorting determinants exist in soybean 11S globulin. Plant Cell 18 1253–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masclaux FG, Galaud JP, Pont-Lezica R (2005) The riddle of the plant vacuolar sorting receptors. Protoplasma 226 103–108 [DOI] [PubMed] [Google Scholar]
- Matsuoka K, Nakamura K (1991) Propeptide of a precursor to a plant vacuolar protein required for vacuolar targeting. Proc Natl Acad Sci USA 88 834–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchers LS, Sela-Buurlage MB, Vloemans SA, Woloshuk CP, Van Roekel JS, Pen J, van den Elzen PJ, Cornelissen BJ (1993) Extracellular targeting of the vacuolar tobacco proteins AP24, chitinase and beta-1,3-glucanase in transgenic plants. Plant Mol Biol 21 583–593 [DOI] [PubMed] [Google Scholar]
- Memon AR (2004) The role of ADP-ribosylation factor and SAR1 in vesicular trafficking in plants. Biochim Biophys Acta 1664 9–30 [DOI] [PubMed] [Google Scholar]
- Miao Y, Yan PK, Kim H, Hwang I, Jiang L (2006) Localization of green fluorescent protein fusions with the seven Arabidopsis vacuolar sorting receptors to prevacuolar compartments in tobacco BY-2 cells. Plant Physiol 142 945–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo B, Tse YC, Jiang L (2006) Plant prevacuolar/endosomal compartments. Int Rev Cytol 253 95–129 [DOI] [PubMed] [Google Scholar]
- Nakatsu F, Ohno H (2003) Adaptor protein complexes as the key regulators of protein sorting in the post-Golgi network. Cell Struct Funct 28 419–429 [DOI] [PubMed] [Google Scholar]
- Neuhaus JM, Pietrzak M, Boller T (1994) Mutation analysis of the C-terminal vacuolar targeting peptide of tobacco chitinase: low specificity of the sorting system, and gradual transition between intracellular retention and secretion into the extracellular space. Plant J 5 45–54 [DOI] [PubMed] [Google Scholar]
- Neuhaus JM, Rogers JC (1998) Sorting of proteins to vacuoles in plant cells. Plant Mol Biol 38 127–144 [PubMed] [Google Scholar]
- Neuhaus JM, Sticher L, Meins F Jr, Boller T (1991) A short C-terminal sequence is necessary and sufficient for the targeting of chitinases to the plant vacuole. Proc Natl Acad Sci USA 88 10362–10366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell-Litwa K, Seong E, Burmeister M, Faundez V (2007) Neuronal and non-neuronal functions of the AP-3 sorting machinery. J Cell Sci 120 531–541 [DOI] [PubMed] [Google Scholar]
- Ni X, Canuel M, Morales CR (2006) The sorting and trafficking of lysosomal proteins. Histol Histopathol 21 899–913 [DOI] [PubMed] [Google Scholar]
- Nishizawa K, Maruyama N, Satoh R, Fuchikami Y, Higasa T, Utsumi S (2003) A C-terminal sequence of soybean beta-conglycinin alpha′ subunit acts as a vacuolar sorting determinant in seed cells. Plant J 34 647–659 [DOI] [PubMed] [Google Scholar]
- Odorizzi G, Cowles CR, Emr SD (1998) The AP-3 complex: a coat of many colours. Trends Cell Biol 8 282–288 [DOI] [PubMed] [Google Scholar]
- Oliviusson P, Heinzerling O, Hillmer S, Hinz G, Tse YC, Jiang L, Robinson DG (2006) Plant retromer, localized to the prevacuolar compartment and microvesicles in Arabidopsis, may interact with vacuolar sorting receptors. Plant Cell 18 1239–1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otegui MS, Herder R, Schulze J, Jung R, Staehelin LA (2006) The proteolytic processing of seed storage proteins in Arabidopsis embryo cells starts in the multivesicular bodies. Plant Cell 18 2567–2581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oufattole M, Park JH, Poxleitner M, Jiang L, Rogers JC (2005) Selective membrane protein internalization accompanies movement from the endoplasmic reticulum to the protein storage vacuole pathway in Arabidopsis. Plant Cell 17 3066–3080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris N, Neuhaus JM (2002) BP-80 as vacuolar sorting receptor. Plant Mol Biol 50 903–914 [DOI] [PubMed] [Google Scholar]
- Paris N, Rogers SW, Jiang L, Kirsch T, Beevers L, Phillips TE, Rogers JC (1997) Molecular cloning and further characterization of a probable plant vacuolar sorting receptor. Plant Physiol 115 29–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, Oufattole M, Rogers JC (2007) Golgi-mediated vacuolar sorting in plant cells: RMR proteins are sorting receptors for the protein aggregation/membrane internalization pathway. Plant Sci 172 728–745 [Google Scholar]
- Park M, Kim SJ, Vitale A, Hwang I (2004) Identification of the protein storage vacuole and protein targeting to the vacuole in leaf cells of three plant species. Plant Physiol 134 625–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M, Lee D, Lee GJ, Hwang I (2005) AtRMR1 functions as a cargo receptor for protein trafficking to the protein storage vacuole. J Cell Biol 170 757–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petruccelli S, Molina MI, Lareu FJ, Circosta A (2007) Two short sequences from amaranth 11S globulin are sufficient to target green fluorescent protein and beta-glucuronidase to vacuoles in Arabidopsis cells. Plant Physiol Biochem 45 400–409 [DOI] [PubMed] [Google Scholar]
- Pimpl P, Hanton SL, Taylor JP, Pinto-daSilva LL, Denecke J (2003) The GTPase ARF1p controls the sequence-specific vacuolar sorting route to the lytic vacuole. Plant Cell 15 1242–1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper RC, Bryant NJ, Stevens TH (1997) The membrane protein alkaline phosphatase is delivered to the vacuole by a route that is distinct from the VPS-dependent pathway. J Cell Biol 138 531–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper RC, Luzio JP (2007) Ubiquitin-dependent sorting of integral membrane proteins for degradation in lysosomes. Curr Opin Cell Biol 19 459–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuss ML, Santos-Serna J, Falbel TG, Bednarek SY, Nielsen E (2004) The Arabidopsis Rab GTPase RabA4b localizes to the tips of growing root hair cells. Plant Cell 16 1589–1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport TA, Rolls MM, Jungnickel B (1996) Approaching the mechanism of protein transport across the ER membrane. Curr Opin Cell Biol 8 499–504 [DOI] [PubMed] [Google Scholar]
- Reczek D, Schwake M, Schröder J, Hughes H, Blanz J, Jin X, Brondyk W, Van Patten S, Edmunds T, Saftig P (2007) LIMP-2 is a receptor for lysosomal mannose-6-phosphate-independent targeting of beta-glucocerebrosidase. Cell 131 770–783 [DOI] [PubMed] [Google Scholar]
- Robatzek S (2007) Vesicle trafficking in plant immune responses. Cell Microbiol 9 1–8 [DOI] [PubMed] [Google Scholar]
- Robinson DG, Herranz MC, Bubeck J, Pepperkok R, Ritzenthaler C (2007) Membrane dynamics in the early secretory pathway. Crit Rev Plant Sci 26 199–225 [Google Scholar]
- Robinson DG, Oliviusson P, Hinz G (2005) Protein sorting to the storage vacuoles of plants: a critical appraisal. Traffic 6 615–625 [DOI] [PubMed] [Google Scholar]
- Robinson MS (2004) Adaptable adaptors for coated vesicles. Trends Cell Biol 14 167–174 [DOI] [PubMed] [Google Scholar]
- Roth MG (1999) Snapshots of ARF1: implications for mechanisms of activation and inactivation. Cell 97 149–152 [DOI] [PubMed] [Google Scholar]
- Saalbach G, Jung R, Kunze G, Saalbach I, Adler K, Müntz K (1991) Different legumin protein domains act as vacuolar targeting signals. Plant Cell 3 695–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderfoot AA, Ahmed SU, Marty-Mazars D, Rapoport I, Kirchhausen T, Marty F, Raikhel NV (1998) A putative vacuolar cargo receptor partially colocalizes with AtPEP12p on a prevacuolar compartment in Arabidopsis roots. Proc Natl Acad Sci USA 95 9920–9925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderfoot AA, Kovaleva V, Bassham DC, Raikhel NV (2001) Interactions between syntaxins identify at least five SNARE complexes within the Golgi/prevacuolar system of the Arabidopsis cell. Mol Biol Cell 12 3733–3743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanmartín M, Ordóñez A, Sohn EJ, Robert S, Sánchez-Serrano JJ, Surpin MA, Raikhel NV, Rojo E (2007) Divergent functions of VTI12 and VTI11 in trafficking to storage and lytic vacuoles in Arabidopsis. Proc Natl Acad Sci USA 104 3645–3650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman MNJ, Marcusson EG, Cereghino JL, Emr SD (1997) Endosome to Golgi retrieval of the vacuolar protein sorting receptor, Vps10p, requires the function of VPS29, VPS30 and VPS35 gene products. J Cell Biol 137 79–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiba T, Kawasaki M, Takatsu H, Nogi T, Matsugaki N, Igarashi N, Suzuki M, Kato R, Nakayama K, Wakatsuki S (2003) Molecular mechanism of membrane recruitment of GGA by ARF in lysosomal protein transport. Nat Struct Biol 10 386–393 [DOI] [PubMed] [Google Scholar]
- Shimada T, Fuji K, Tamura K, Kondo M, Nishimura M, Hara-Nishimura I (2003) Vacuolar sorting receptor for seed storage proteins in Arabidopsis thaliana. Proc Natl Acad Sci USA 100 16095–16100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada T, Koumoto Y, Li L, Yamazaki M, Kondo M, Nishimura M, Hara-Nishimura I (2006) AtVPS29, a putative component of a retromer complex, is required for the efficient sorting of seed storage proteins. Plant Cell Physiol 47 1187–1194 [DOI] [PubMed] [Google Scholar]
- Simões I, Faro C (2004) Structure and function of plant aspartic proteinases. Eur J Biochem 271 2067–2075 [DOI] [PubMed] [Google Scholar]
- Sly WS, Fisher HD (1982) The phosphomannosyl recognition system for intracellular and intercellular transport of lysosomal enzymes. J Cell Biochem 18 67–85 [DOI] [PubMed] [Google Scholar]
- Sohn EJ, Kim ES, Zhao M, Kim SJ, Kim H, Kim YW, Lee YJ, Hillmer S, Sohn U, Jiang L, et al (2003) Rha1, an Arabidopsis Rab5 homolog, plays a critical role in the vacuolar trafficking of soluble cargo proteins. Plant Cell 15 1057–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn EJ, Rojas-Pierce M, Pan S, Carter C, Serrano-Mislata A, Madueño F, Rojo E, Surpin M, Raikhel NV (2007) The shoot meristem identity gene TFL1 is involved in flower development and trafficking to the protein storage vacuole. Proc Natl Acad Sci USA 104 18801–18806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Lee MH, Lee GJ, Yoo CM, Hwang I (2006) Arabidopsis EPSIN1 plays an important role in vacuolar trafficking of soluble cargo proteins in plant cells via interactions with clathrin, AP-1, VTI11, and VSR1. Plant Cell 18 2258–2274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staab JF, Ginkel DL, Rosenberg GB, Munford RS (1994) A saposin-like domain influences the intracellular localization, stability, and catalytical activity of human acyloxyacyl hydrolase. J Biol Chem 269 23736–23742 [PubMed] [Google Scholar]
- Sticher L, Hofsteenge J, Milani A, Neuhaus JM, Meins F Jr (1992) Vacuolar chitinases of tobacco: a new class of hydroxyproline-containing proteins. Science 257 655–657 [DOI] [PubMed] [Google Scholar]
- Surpin M, Raikhel N (2004) Traffic jams affect plant development and signal transduction. Nat Rev Mol Cell Biol 5 100–109 [DOI] [PubMed] [Google Scholar]
- Surpin M, Zheng H, Morita MT, Saito C, Avila E, Blakeslee JJ, Bandyopadhyay A, Kovaleva V, Carter D, Murphy A, et al (2003) The VTI family of SNARE proteins is necessary for plant viability and mediates different protein transport pathways. Plant Cell 15 2885–2899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi M, Ueda T, Yahara N, Nakano A (2002) Arf1 GTPase plays roles in the protein traffic between the endoplasmic reticulum and the Golgi apparatus in tobacco and Arabidopsis cultured cells. Plant J 31 499–515 [DOI] [PubMed] [Google Scholar]
- Tang BL, Wang Y, Ong YS, Hong W (2005) COPII and exit from the endoplasmic reticulum. Biochim Biophys Acta 1744 293–303 [DOI] [PubMed] [Google Scholar]
- Thomine S, Lelièvre F, Debarbieux E, Schroeder JI, Barbier-Brygoo H (2003) AtNRAMP3, a multispecific vacuolar metal transporter involved in plant responses to iron deficiency. Plant J 34 685–695 [DOI] [PubMed] [Google Scholar]
- Tormakangas K, Hadlington J, Pimpl P, Hillmer S, Brandizzi F, Teeri T, Denecke J (2001) A vacuolar sorting domain may also influence the way in which proteins leave the endoplasmic reticulum. Plant Cell 13 2021–2032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub LM (2005) Common principles in clathrin-mediated sorting at the Golgi and the plasma membrane. Biochim Biophys Acta 1744 415–437 [DOI] [PubMed] [Google Scholar]
- Tse YC, Mo B, Hillmer S, Zhao M, Lo SW, Robinson DG, Jiang L (2004) Identification of multivesicular bodies as prevacuolar compartments in Nicotiana tabacum BY-2 cells. Plant Cell 16 672–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungewickell EJ, Hinrichsen L (2007) Endocytosis: clathrin-mediated membrane budding. Curr Opin Cell Biol 19 417–425 [DOI] [PubMed] [Google Scholar]
- Vitale A, Chrispeels MJ (1992) Sorting of proteins to the vacuoles of plant cells. Bioessays 14 151–160 [DOI] [PubMed] [Google Scholar]
- Vitale A, Hinz G (2005) Sorting of proteins to storage vacuoles: how many mechanisms? Trends Plant Sci 10 316–323 [DOI] [PubMed] [Google Scholar]
- von der Fecht-Bartenbach J, Bogner M, Krebs M, Stierhof YD, Schumacher K, Ludewig U (2007) Function of the anion transporter AtCLC-d in the trans-Golgi network. Plant J 50 466–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Li Y, Lo SW, Hillmer S, Sun SS, Robinson DG, Jiang L (2007) Protein mobilization in germinating mung bean seeds involves vacuolar sorting receptors and multivesicular bodies. Plant Physiol 143 1628–1639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte JR, Munro S (2001) A yeast homolog of the mammalian mannose 6-phosphate receptors contributes to the sorting of vacuolar hydrolases. Curr Biol 11 1074–1078 [DOI] [PubMed] [Google Scholar]
- Yamazaki M, Shimada T, Takahashi H, Tamura K, Kondo M, Nishimura M, Hara-Nishimura I (2008) Arabidopsis VPS35, a retromer component, is required for vacuolar protein sorting and involved in plant growth and leaf senescence. Plant Cell Physiol 49 142–156 [DOI] [PubMed] [Google Scholar]
- Zheng H, Camacho L, Wee E, Batoko H, Legen J, Leaver CJ, Malhó R, Hussey PJ, Moore I (2005) A Rab-E GTPase mutant acts downstream of the Rab-D subclass in biosynthetic membrane traffic to the plasma membrane in tobacco leaf epidermis. Plant Cell 17 2020–2036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, von Mollard GF, Kovaleva V, Stevens TH, Raikhel NV (1999) The plant vesicle-associated SNARE AtVTI1a likely mediates vesicle transport from the trans-Golgi network to the prevacuolar compartment. Mol Biol Cell 10 2251–2264 [DOI] [PMC free article] [PubMed] [Google Scholar]