Abstract
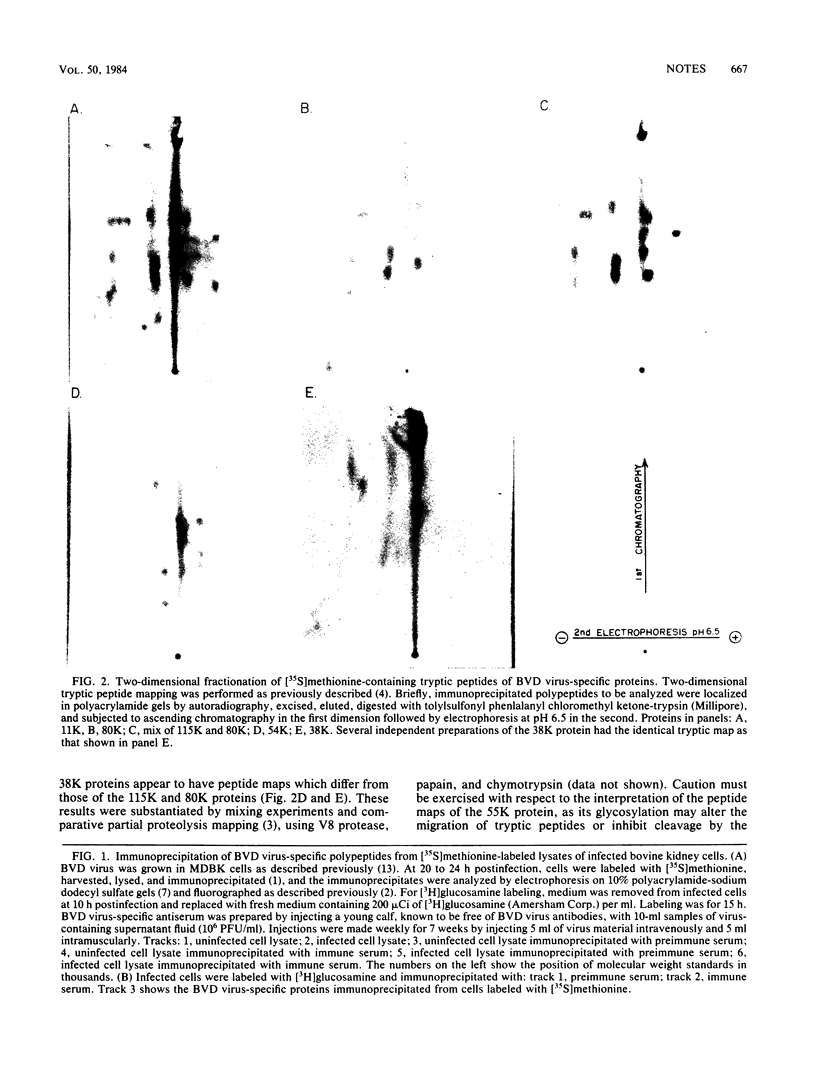
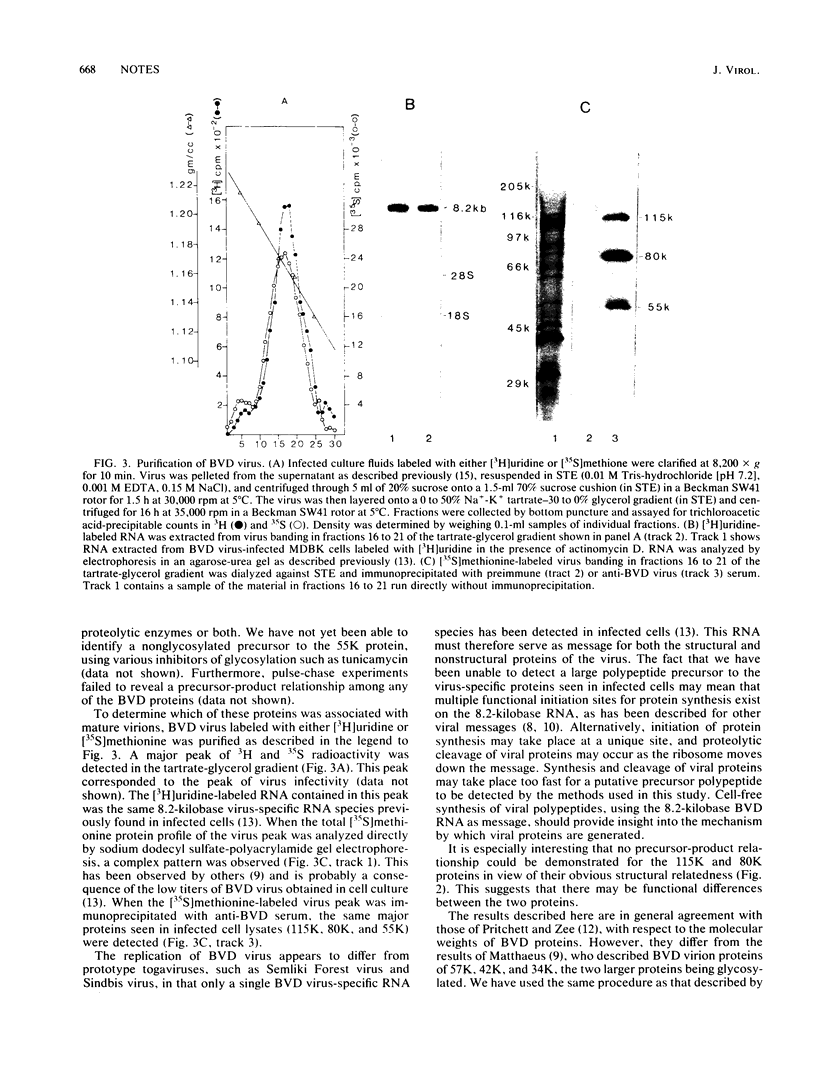
Virus-specific proteins were examined in cultured cells infected with bovine viral diarrhea virus. By using antisera obtained from virus-infected animals, three major virus-specific polypeptides with molecular weights of 115,000 (115K), 80K, and 55K were observed. Minor proteins of 45,000 and 38,000 daltons were also noted. Tryptic peptide mapping indicated that the 115K and the 80K polypeptides were structurally related. The 55K protein was glycosylated and appeared not to be related to the 115K and 80K proteins. Pulse-chase experiments failed to demonstrate any procursor-product relationship among any of these proteins, and all three polypeptides were found in purified virion preparations. The significance of these findings with respect to the replication of bovine viral diarrhea virus is discussed.
Full text
PDF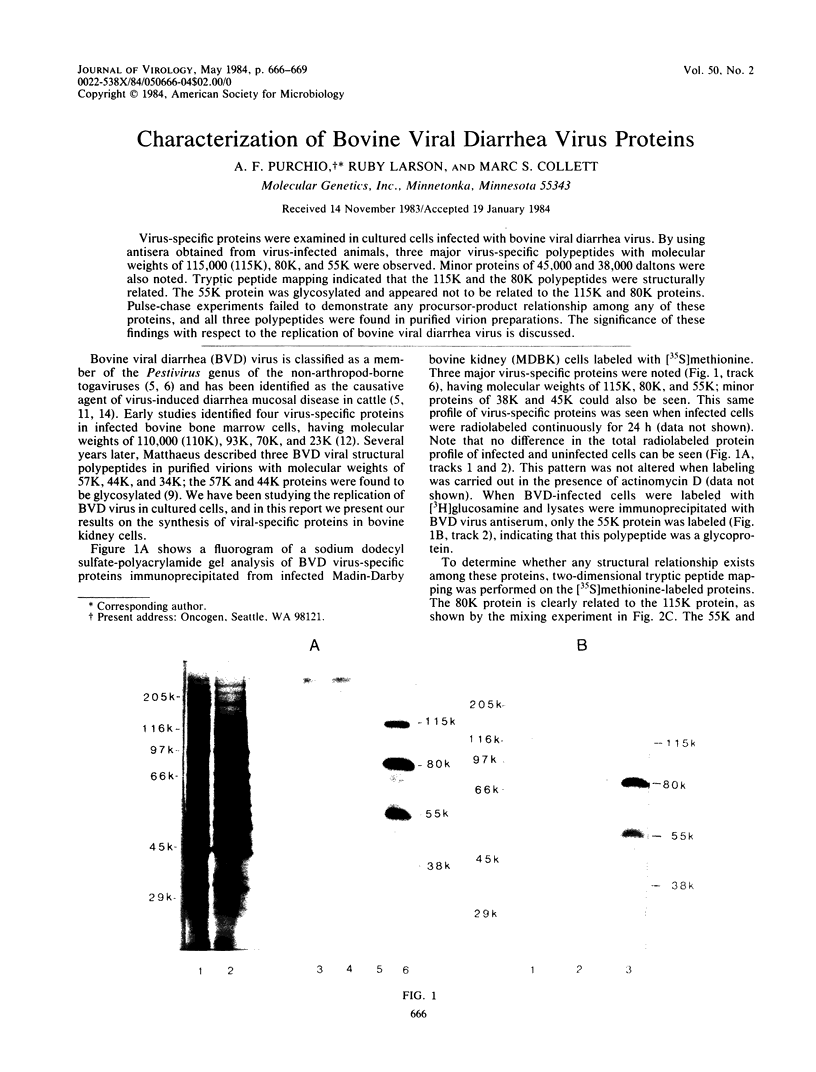

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brugge J. S., Erikson R. L. Identification of a transformation-specific antigen induced by an avian sarcoma virus. Nature. 1977 Sep 22;269(5626):346–348. doi: 10.1038/269346a0. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Collett M. S., Erikson E., Erikson R. L. Structural analysis of the avian sarcoma virus transforming protein: sites of phosphorylation. J Virol. 1979 Feb;29(2):770–781. doi: 10.1128/jvi.29.2.770-781.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marsden H. S., Haarr L., Preston C. M. Processing of herpes simplex virus proteins and evidence that translation of thymidine kinase mRNA is initiated at three separate AUG codons. J Virol. 1983 May;46(2):434–445. doi: 10.1128/jvi.46.2.434-445.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthaeus W. Detection of three polypeptides in preparations of bovine viral diarrhoea virus. Arch Virol. 1979;59(4):299–305. doi: 10.1007/BF01317470. [DOI] [PubMed] [Google Scholar]
- Perez-Bercoff R., Kaempfer R. Genomic RNA of mengovirus V. Recognition of common features by ribosomes and eucaryotic initiation factor 2. J Virol. 1982 Jan;41(1):30–41. doi: 10.1128/jvi.41.1.30-41.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchett R. F., Zee Y. C. Structural proteins of bovine viral diarrhea virus. Am J Vet Res. 1975 Dec;36(12):1731–1734. [PubMed] [Google Scholar]
- Purchio A. F., Larson R., Collett M. S. Characterization of virus-specific RNA synthesized in bovine cells infected with bovine viral diarrhea virus. J Virol. 1983 Oct;48(1):320–324. doi: 10.1128/jvi.48.1.320-324.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volenec F. J., Sheffy B. E., Baker J. A. Immunobiologic hetertypic activity associated with viral and soluble components of bovine virus diarrhea virus. Arch Gesamte Virusforsch. 1972;36(3):275–283. doi: 10.1007/BF01249858. [DOI] [PubMed] [Google Scholar]