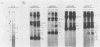Abstract
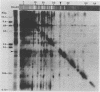
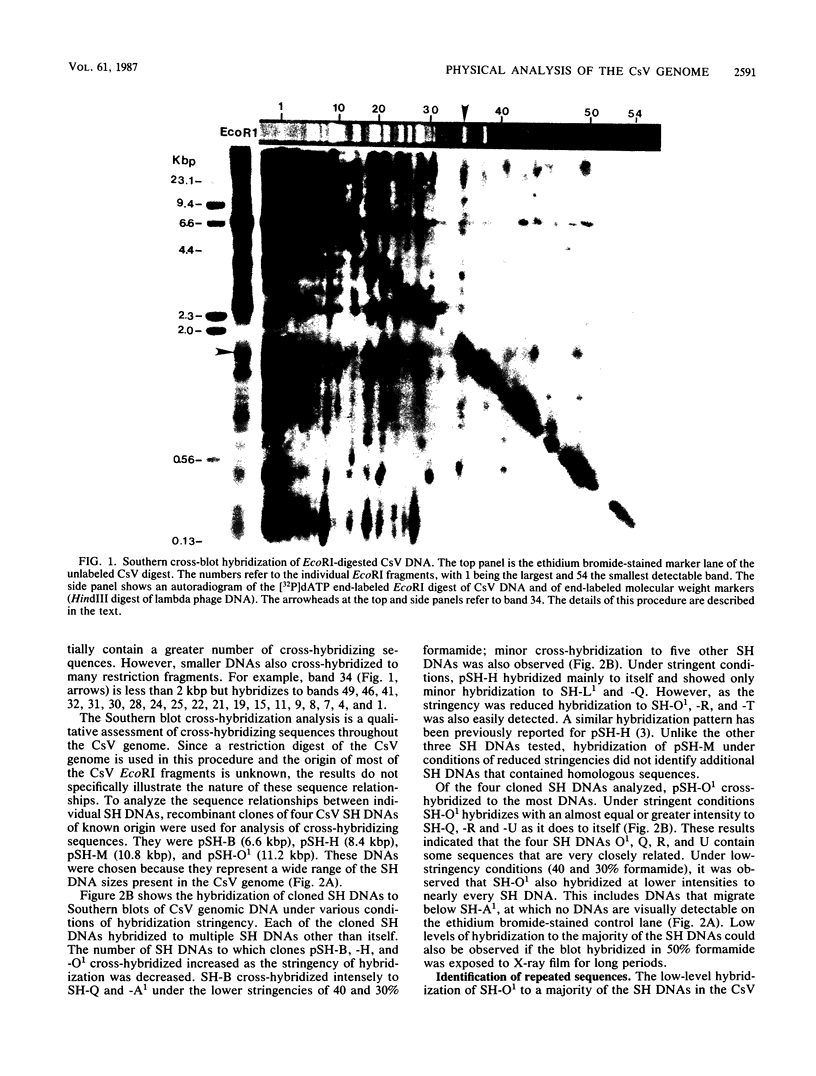
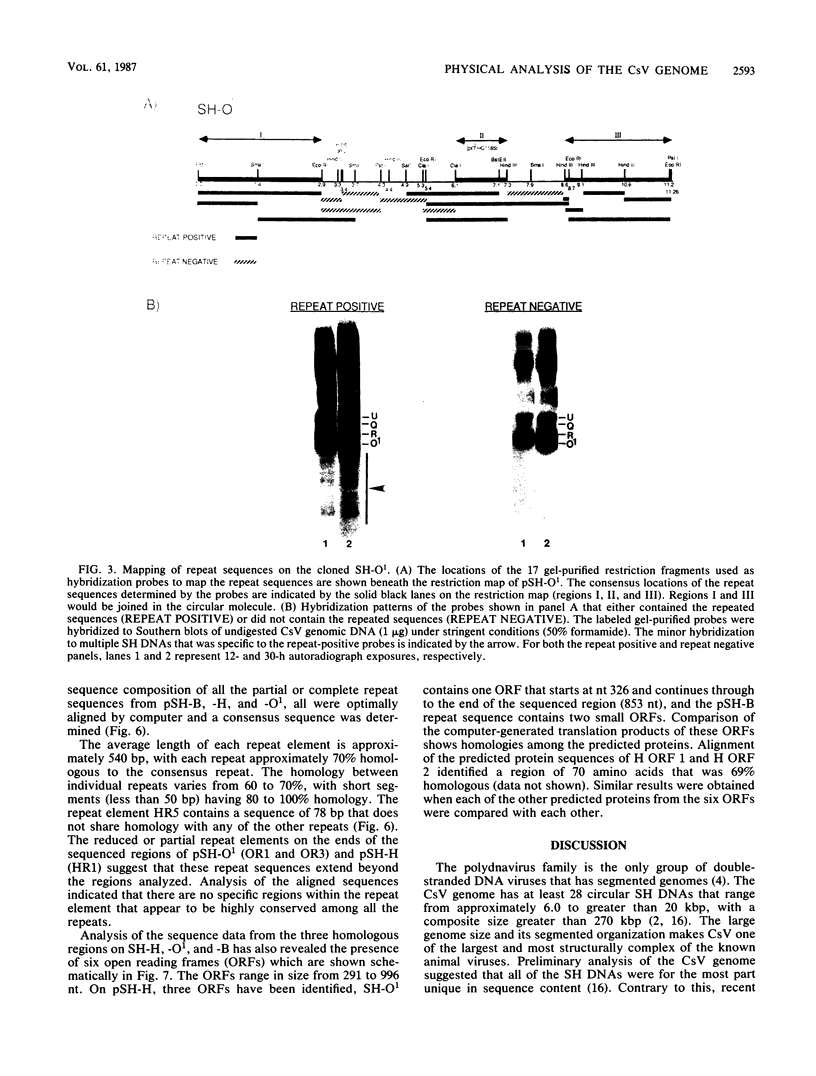
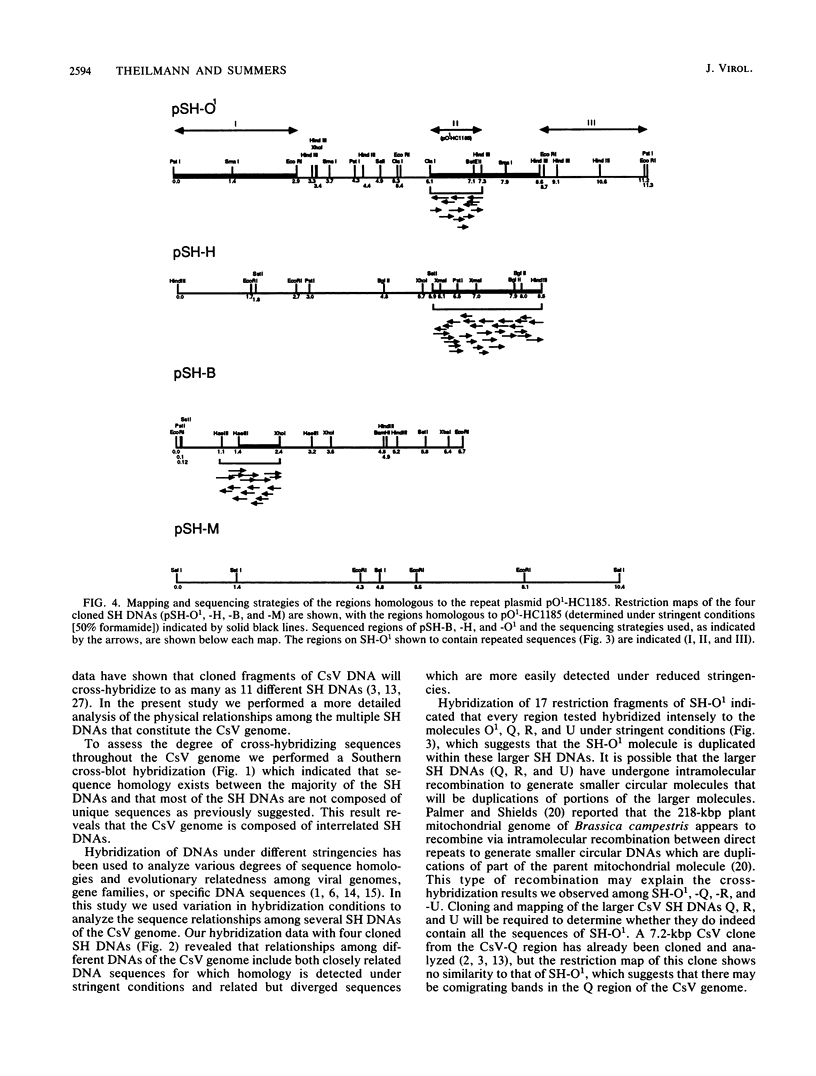
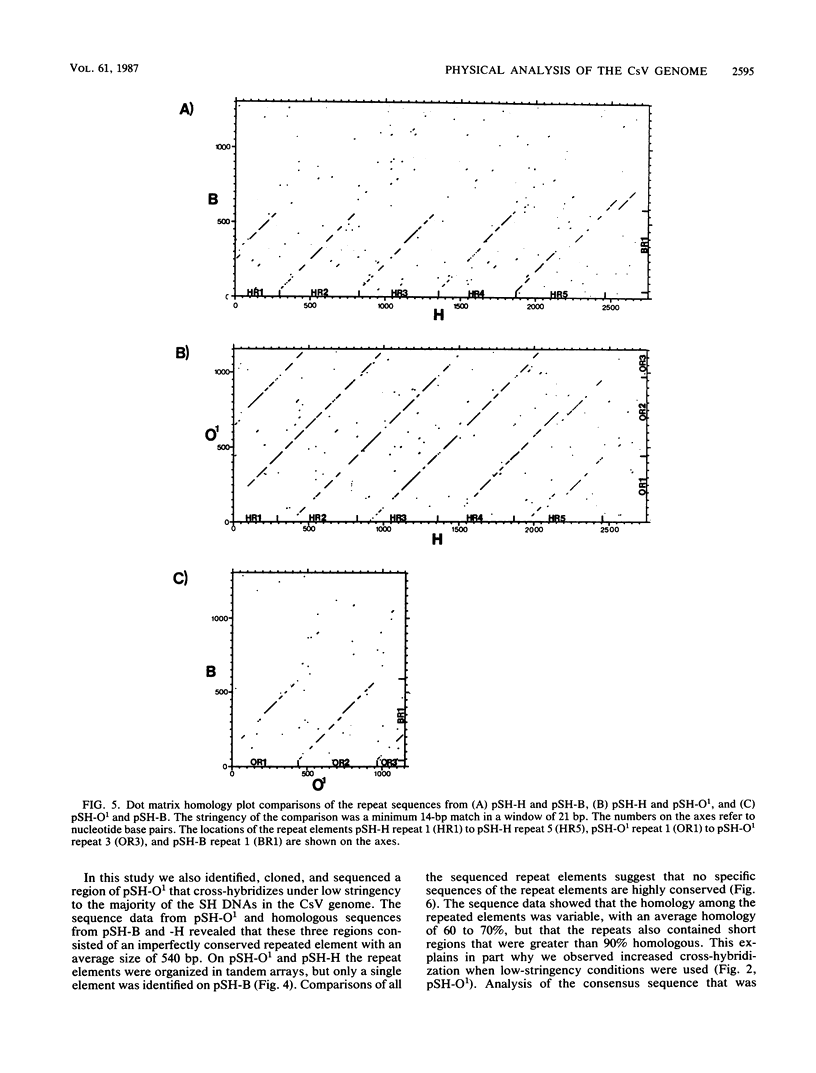
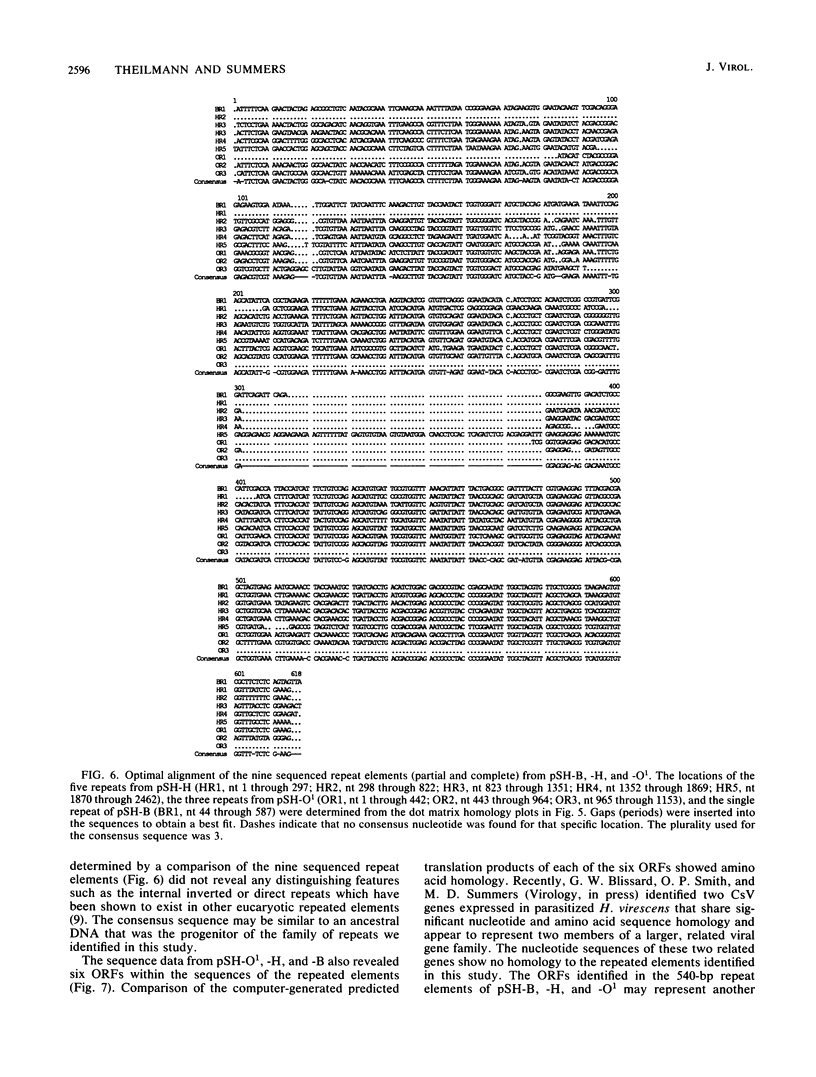
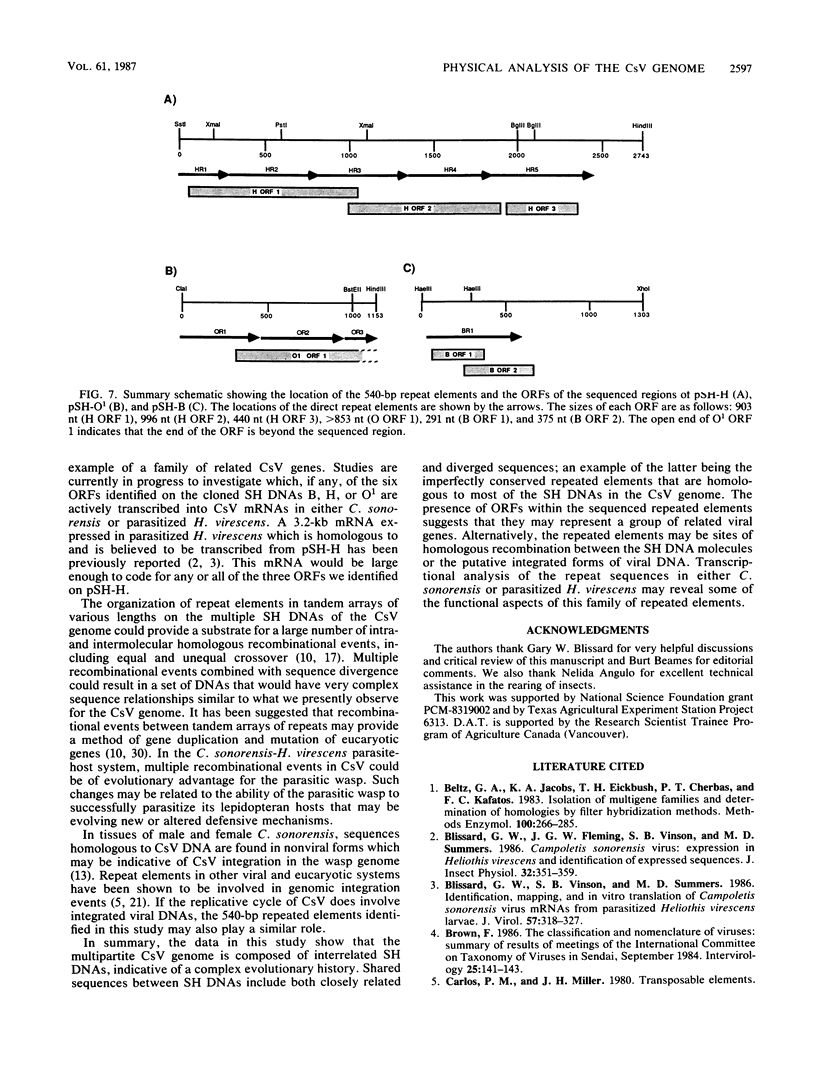
This report is an analysis of cross-hybridizing sequences found within the 28 superhelical (SH) DNAs of the multipartite genome of the polydnavirus Campoletis sonorensis virus (CsV). A Southern cross-blot hybridization analysis showed that the majority of CsV EcoRI restriction fragments cross-hybridize to multiple EcoRI fragments. These sequence homologies were analyzed by hybridizing recombinant clones of the CsV SH DNAs B, H, M, and O1 to Southern blots of undigested CsV DNA, using different hybridization stringencies. The results indicated that homologous regions among the SH DNAs include closely related sequences that are detectable under stringent conditions and related but more diverged sequences which are only detectable under reduced stringencies. A sequence that hybridized to the majority of the CsV SH DNAs was identified and subcloned from the SH DNAs O1, H, and B. Nucleotide sequence data revealed that these homologous regions contained a family of imperfectly conserved repeated elements. These repeat elements were arranged singly or in direct tandem arrays and had an average length of 540 base pairs. Within the sequenced regions that contained the repeated elements six putative open reading frames were identified. These results show that the CsV genome consists of SH DNAs with complex sequence interrelationships that may have arisen due to multiple recombinational events.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beltz G. A., Jacobs K. A., Eickbush T. H., Cherbas P. T., Kafatos F. C. Isolation of multigene families and determination of homologies by filter hybridization methods. Methods Enzymol. 1983;100:266–285. doi: 10.1016/0076-6879(83)00061-0. [DOI] [PubMed] [Google Scholar]
- Blissard G. W., Vinson S. B., Summers M. D. Identification, Mapping, and In Vitro Translation of Campoletis sonorensis Virus mRNAs from Parasitized Heliothis virescens Larvae. J Virol. 1986 Jan;57(1):318–327. doi: 10.1128/jvi.57.1.318-327.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochran M. A., Faulkner P. Location of Homologous DNA Sequences Interspersed at Five Regions in the Baculovirus AcMNPV Genome. J Virol. 1983 Mar;45(3):961–970. doi: 10.1128/jvi.45.3.961-970.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale R. M., McClure B. A., Houchins J. P. A rapid single-stranded cloning strategy for producing a sequential series of overlapping clones for use in DNA sequencing: application to sequencing the corn mitochondrial 18 S rDNA. Plasmid. 1985 Jan;13(1):31–40. doi: 10.1016/0147-619x(85)90053-8. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edson K. M., Vinson S. B., Stoltz D. B., Summers M. D. Virus in a parasitoid wasp: suppression of the cellular immune response in the parasitoid's host. Science. 1981 Feb 6;211(4482):582–583. doi: 10.1126/science.7455695. [DOI] [PubMed] [Google Scholar]
- Fleming J. A., Blissard G. W., Summers M. D., Vinson S. B. Expression of Campoletis sonorensis Virus in the Parasitized Host, Heliothis virescens. J Virol. 1983 Oct;48(1):74–78. doi: 10.1128/jvi.48.1.74-78.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming J. A., Summers M. D. Campoletis sonorensis Endoparasitic Wasps Contain Forms of C. sonorensis Virus DNA Suggestive of Integrated and Extrachromosomal Polydnavirus DNAs. J Virol. 1986 Feb;57(2):552–562. doi: 10.1128/jvi.57.2.552-562.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howley P. M., Israel M. A., Law M. F., Martin M. A. A rapid method for detecting and mapping homology between heterologous DNAs. Evaluation of polyomavirus genomes. J Biol Chem. 1979 Jun 10;254(11):4876–4883. [PubMed] [Google Scholar]
- Krell P. J., Summers M. D., Vinson S. B. Virus with a Multipartite Superhelical DNA Genome from the Ichneumonid Parasitoid Campoletis sonorensis. J Virol. 1982 Sep;43(3):859–870. doi: 10.1128/jvi.43.3.859-870.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maizel J. V., Jr, Lenk R. P. Enhanced graphic matrix analysis of nucleic acid and protein sequences. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7665–7669. doi: 10.1073/pnas.78.12.7665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panganiban A. T. Retroviral DNA integration. Cell. 1985 Aug;42(1):5–6. doi: 10.1016/s0092-8674(85)80092-1. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. E., Summers M. D. The bidirectional transfer of DNA and RNA to nitrocellulose or diazobenzyloxymethyl-paper. Anal Biochem. 1980 Nov 15;109(1):123–129. doi: 10.1016/0003-2697(80)90019-6. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stoltz D. B., Vinson S. B. Viruses and parasitism in insects. Adv Virus Res. 1979;24:125–171. doi: 10.1016/s0065-3527(08)60393-0. [DOI] [PubMed] [Google Scholar]
- Theilmann D. A., Summers M. D. Molecular analysis of Campoletis sonorensis virus DNA in the lepidopteran host Heliothis virescens. J Gen Virol. 1986 Sep;67(Pt 9):1961–1969. doi: 10.1099/0022-1317-67-9-1961. [DOI] [PubMed] [Google Scholar]
- Wilson A. C., Carlson S. S., White T. J. Biochemical evolution. Annu Rev Biochem. 1977;46:573–639. doi: 10.1146/annurev.bi.46.070177.003041. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]