Abstract
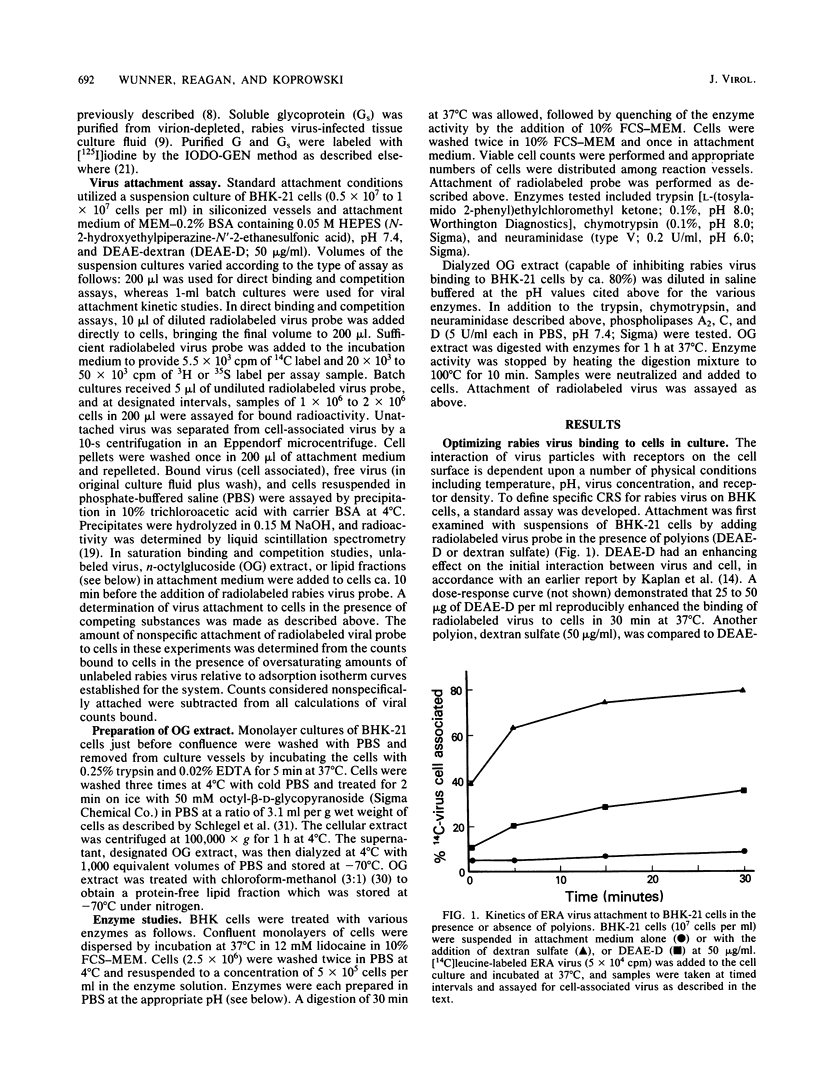
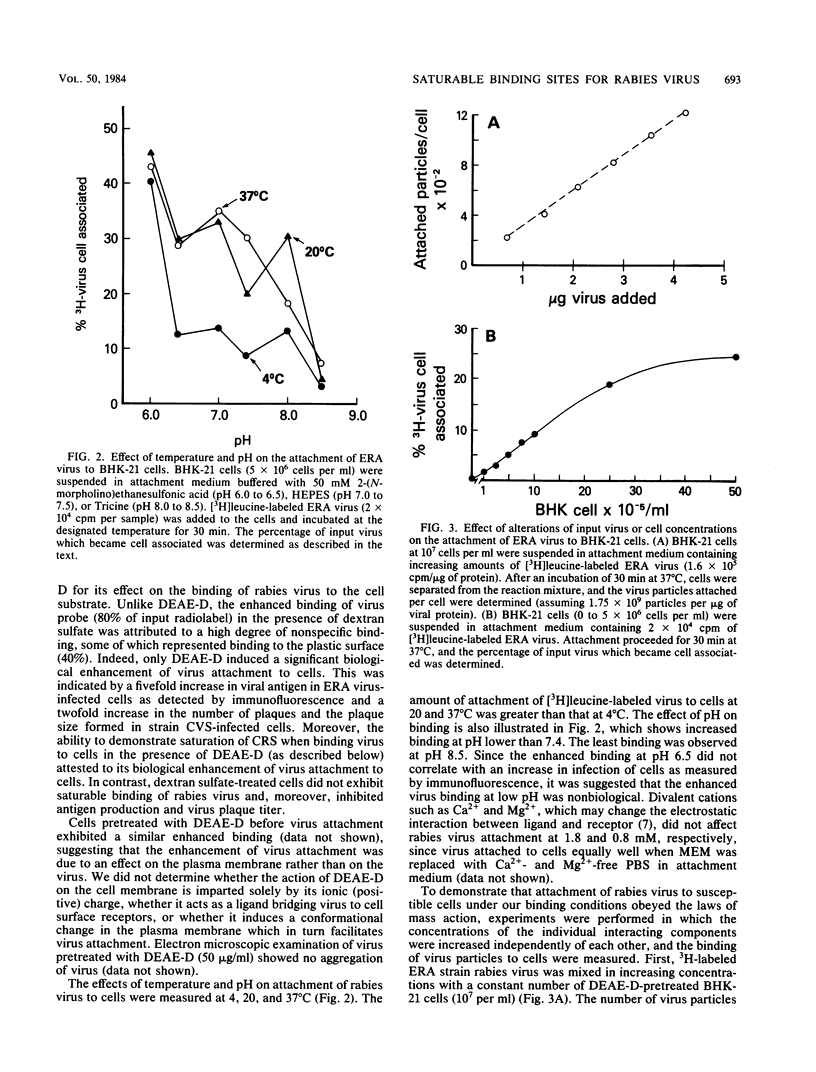
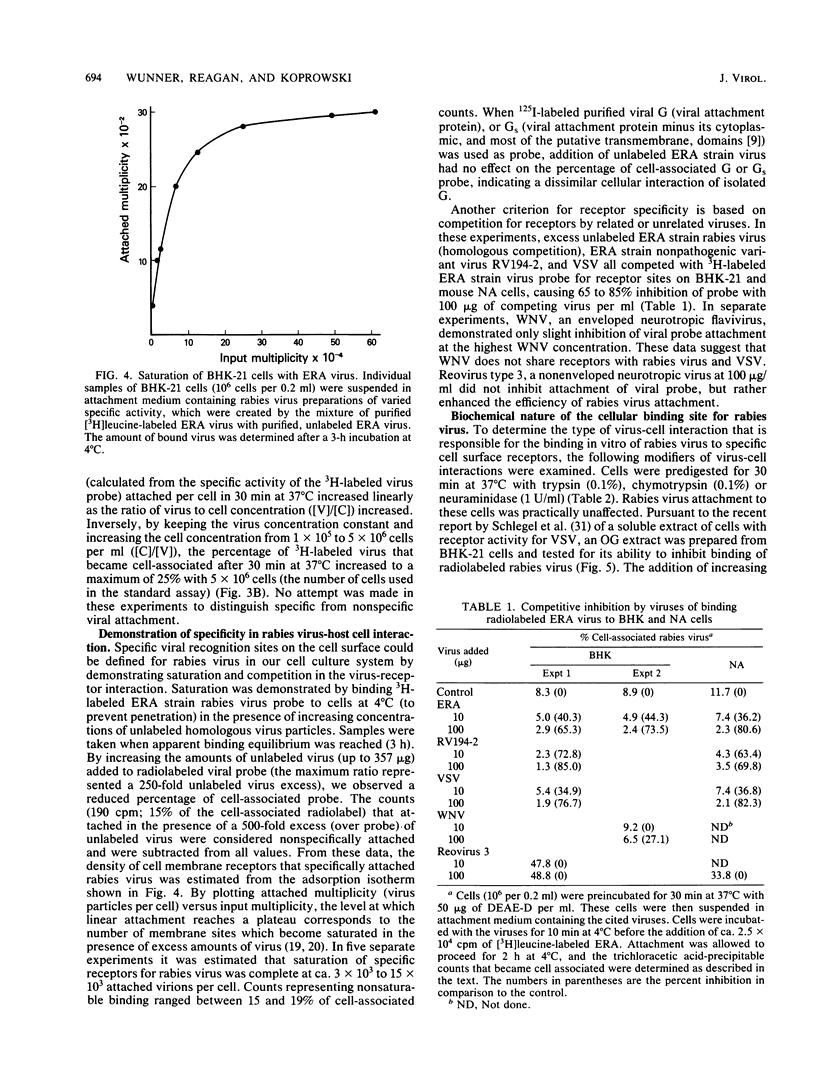
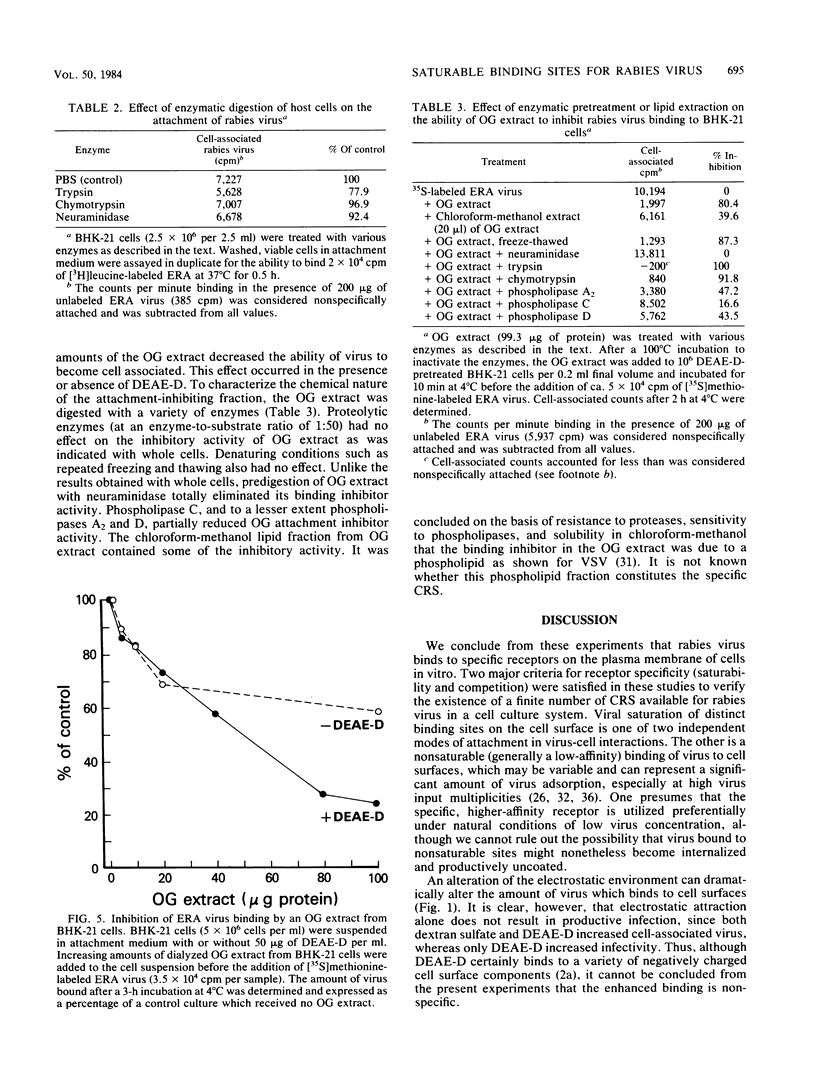
A specific, saturable receptor for rabies virus was analyzed on cultured cells of neural or non-neural origin. Viral attachment kinetics were enhanced by DEAE-dextran, an effect which in turn enhanced the apparent infectivity of the virus inoculum. Under optimized conditions, the attachment of metabolically labeled ERA strain rabies virus obeyed the laws of mass action, whereby the amount of virus bound to cells varied proportionally with the concentration of cells or virus. Attachment was sensitive to changes of temperature and pH, did not require divalent cations such as Mg2+ or Ca2+, and occurred despite prior treatment of cells with proteolytic or sialic acid-specific enzymes. Saturation of the cell surface with rabies virus could be accomplished with 3 X 10(3) to 15 X 10(3) attached virions per cell. Competition for the rabies receptor occurred with rabies nonpathogenic variant virus, RV194 -2, and vesicular stomatitis virus. Reovirus type 3, another neurotropic virus, failed to inhibit rabies virus binding, and West Nile virus only slightly inhibited rabies virus binding, suggesting independent cellular receptors were recognized by these viruses. Isolated rabies virus glycoprotein failed to compete in an equivalent manner. However, solubilization of BHK-21 cells with octylglucoside yielded a chloroform-methanol-soluble extract which blocked rabies virus attachment. The binding inhibition activity of this extract was resistant to proteases but could be destroyed by phospholipases and neuraminidase, suggesting a phospholipid or glycolipid component at the receptor site. These data provide evidence for a rhabdovirus-common mechanism for cellular attachment to cells in culture.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altrock B. W., Arthur L. O., Massey R. J., Schochetman G. Common surface receptors on both mouse and rat cells distinguish different classes of mouse mammary tumor viruses. Virology. 1981 Mar;109(2):257–266. doi: 10.1016/0042-6822(81)90497-9. [DOI] [PubMed] [Google Scholar]
- Anilionis A., Wunner W. H., Curtis P. J. Structure of the glycoprotein gene in rabies virus. Nature. 1981 Nov 19;294(5838):275–278. doi: 10.1038/294275a0. [DOI] [PubMed] [Google Scholar]
- Bailey C. A., Miller D. K., Lenard J. Effects of DEAE-dextran on infection and hemolysis by VSV. Evidence that nonspecific electrostatic interactions mediate effective binding of VSV to cells. Virology. 1984 Feb;133(1):111–118. doi: 10.1016/0042-6822(84)90429-x. [DOI] [PubMed] [Google Scholar]
- Brinton M. A., Fernandez A. V. A replication-efficient mutant of West Nile virus is insensitive to DI particle interference. Virology. 1983 Aug;129(1):107–115. doi: 10.1016/0042-6822(83)90399-9. [DOI] [PubMed] [Google Scholar]
- Clark H. F., Parks N. F., Wunner W. H. Defective interfering particles of fixed rabies viruses: lack of correlation with attenuation or auto-interference in mice. J Gen Virol. 1981 Feb;52(Pt 2):245–258. doi: 10.1099/0022-1317-52-2-245. [DOI] [PubMed] [Google Scholar]
- Dietzschold B., Cox J. H., Schneider L. G., Wiktor T. J., Koprowski H. Isolation and purification of a polymeric form of the glycoprotein of rabies virus. J Gen Virol. 1978 Jul;40(1):131–139. doi: 10.1099/0022-1317-40-1-131. [DOI] [PubMed] [Google Scholar]
- Dietzschold B., Wiktor T. J., Wunner W. H., Varrichio A. Chemical and immunological analysis of the rabies soluble glycoprotein. Virology. 1983 Jan 30;124(2):330–337. doi: 10.1016/0042-6822(83)90349-5. [DOI] [PubMed] [Google Scholar]
- Dietzschold B., Wunner W. H., Wiktor T. J., Lopes A. D., Lafon M., Smith C. L., Koprowski H. Characterization of an antigenic determinant of the glycoprotein that correlates with pathogenicity of rabies virus. Proc Natl Acad Sci U S A. 1983 Jan;80(1):70–74. doi: 10.1073/pnas.80.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLLAND J. J. Receptor affinities as major determinants of enterovirus tissue tropisms in humans. Virology. 1961 Nov;15:312–326. doi: 10.1016/0042-6822(61)90363-4. [DOI] [PubMed] [Google Scholar]
- Iwasaki Y., Wiktor T. J., Koprowski H. Early events of rabies virus replicaton in tissue cultures. An electron microscopic study. Lab Invest. 1973 Feb;28(2):142–148. [PubMed] [Google Scholar]
- Kaplan M. M., Wiktor T. J., Maes R. F., Campbell J. B., Koprowski H. Effect of polyions on the infectivity of rabies virus in tissue culture: construction of a single-cycle growth curve. J Virol. 1967 Feb;1(1):145–151. doi: 10.1128/jvi.1.1.145-151.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentz T. L., Burrage T. G., Smith A. L., Crick J., Tignor G. H. Is the acetylcholine receptor a rabies virus receptor? Science. 1982 Jan 8;215(4529):182–184. doi: 10.1126/science.7053569. [DOI] [PubMed] [Google Scholar]
- Linser P., Bruning H., Armentrout R. W. Specific binding sites for a parvovirus, minute virus of mice, on cultured mouse cells. J Virol. 1977 Oct;24(1):211–221. doi: 10.1128/jvi.24.1.211-221.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonberg-Holm K., Crowell R. L., Philipson L. Unrelated animal viruses share receptors. Nature. 1976 Feb 26;259(5545):679–681. doi: 10.1038/259679a0. [DOI] [PubMed] [Google Scholar]
- Lonberg-Holm K., Korant B. D. Early interaction of rhinoviruses with host cells. J Virol. 1972 Jan;9(1):29–40. doi: 10.1128/jvi.9.1.29-40.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonberg-Holm K., Philipson L. Early interaction between animal viruses and cells. Monogr Virol. 1974;9:1–148. [PubMed] [Google Scholar]
- Markwell M. A., Fox C. F. Surface-specific iodination of membrane proteins of viruses and eucaryotic cells using 1,3,4,6-tetrachloro-3alpha,6alpha-diphenylglycoluril. Biochemistry. 1978 Oct 31;17(22):4807–4817. doi: 10.1021/bi00615a031. [DOI] [PubMed] [Google Scholar]
- McClintock P. R., Billups L. C., Notkins A. L. Receptors for encephalomyocarditis virus on murine and human cells. Virology. 1980 Oct 30;106(2):261–272. doi: 10.1016/0042-6822(80)90249-4. [DOI] [PubMed] [Google Scholar]
- Mifune K., Ohuchi M., Mannen K. Hemolysis and cell fusion by rhabdoviruses. FEBS Lett. 1982 Jan 25;137(2):293–297. doi: 10.1016/0014-5793(82)80370-0. [DOI] [PubMed] [Google Scholar]
- Murphy F. A. Rabies pathogenesis. Arch Virol. 1977;54(4):279–297. doi: 10.1007/BF01314774. [DOI] [PubMed] [Google Scholar]
- Pastan I. H., Willingham M. C. Journey to the center of the cell: role of the receptosome. Science. 1981 Oct 30;214(4520):504–509. doi: 10.1126/science.6170111. [DOI] [PubMed] [Google Scholar]
- Philipson L., Lonberg-Holm K., Pettersson U. Virus-receptor interaction in an adenovirus system. J Virol. 1968 Oct;2(10):1064–1075. doi: 10.1128/jvi.2.10.1064-1075.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. K., Doolittle R. F., Anilionis A., Curtis P. J., Wunner W. H. Homology between the glycoproteins of vesicular stomatitis virus and rabies virus. J Virol. 1982 Jul;43(1):361–364. doi: 10.1128/jvi.43.1.361-364.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. K., Gallione C. J. Nucleotide sequences of the mRNA's encoding the vesicular stomatitis virus G and M proteins determined from cDNA clones containing the complete coding regions. J Virol. 1981 Aug;39(2):519–528. doi: 10.1128/jvi.39.2.519-528.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STOKER M., MACPHERSON I. SYRIAN HAMSTER FIBROBLAST CELL LINE BHK21 AND ITS DERIVATIVES. Nature. 1964 Sep 26;203:1355–1357. doi: 10.1038/2031355a0. [DOI] [PubMed] [Google Scholar]
- Schlegel R., Tralka T. S., Willingham M. C., Pastan I. Inhibition of VSV binding and infectivity by phosphatidylserine: is phosphatidylserine a VSV-binding site? Cell. 1983 Feb;32(2):639–646. doi: 10.1016/0092-8674(83)90483-x. [DOI] [PubMed] [Google Scholar]
- Schlegel R., Willingham M. C., Pastan I. H. Saturable binding sites for vesicular stomatitis virus on the surface of Vero cells. J Virol. 1982 Sep;43(3):871–875. doi: 10.1128/jvi.43.3.871-875.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. L., Tignor G. H. Host cell receptors for two strains of Sindbis virus. Arch Virol. 1980;66(1):11–26. doi: 10.1007/BF01315041. [DOI] [PubMed] [Google Scholar]
- Svensson U., Persson R., Everitt E. Virus-receptor interaction in the adenovirus system I. Identification of virion attachment proteins of the HeLa cell plasma membrane. J Virol. 1981 Apr;38(1):70–81. doi: 10.1128/jvi.38.1.70-81.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardieu M., Epstein R. L., Weiner H. L. Interaction of viruses with cell surface receptors. Int Rev Cytol. 1982;80:27–61. doi: 10.1016/S0074-7696(08)60366-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimmig R. L., Hughes J. V., Kinders R. J., Milenkovic A. G., Johnson T. C. Isolation of the glycoprotein of vesicular stomatitis virus and its binding to cell surfaces. J Gen Virol. 1980 Oct;50(2):279–291. doi: 10.1099/0022-1317-50-2-279. [DOI] [PubMed] [Google Scholar]
- Wiktor T. J., Dietzschold B., Leamnson R. N., Koprowski H. Induction and biological properties of defective interfering particles of rabies virus. J Virol. 1977 Feb;21(2):626–635. doi: 10.1128/jvi.21.2.626-635.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]


