Abstract
The myeloproliferative sarcoma virus (MPSV) induces extensive hematopoietic changes, including spleen foci in adult mice, and transforms fibroblasts in vitro. NRK nonproducer cell lines of MPSV and ts temperature-sensitive mutants were analyzed by restriction enzyme digestion and Southern blotting. EcoRI fragments containing the proviral DNAs of MPSV and two temperature-sensitive mutants and rat cellular sequences homologous to c-mos were molecularly cloned. By comparing restriction enzyme cleavage sites, it was shown that the MPSV genome consists only of sequences related either to Moloney murine leukemia virus or to the c-mos mouse oncogenic sequences. Two regions of fragment heterogeneity were observed: (i) in the defective pol gene, where MPSV and the two cloned temperature-sensitive mutants were different from Moloney murine sarcoma virus and from each other, although MPSV wild-type retained more of the pol gene than any of the Moloney murine sarcoma virus isolates; (ii) in the area 3' to the mos gene, which was identical in MPSV and its temperature-sensitive mutants but different from other Moloney murine sarcoma virus variants. Transfection of cloned MPSV DNA in RAT4 cells and virus rescue on infection with Friend murine leukemia virus yielded MPSV which transformed fibroblasts in vitro and also induced spleen foci in adult mice, thus proving that both properties are coded by the same viral genome.
Full text
PDF



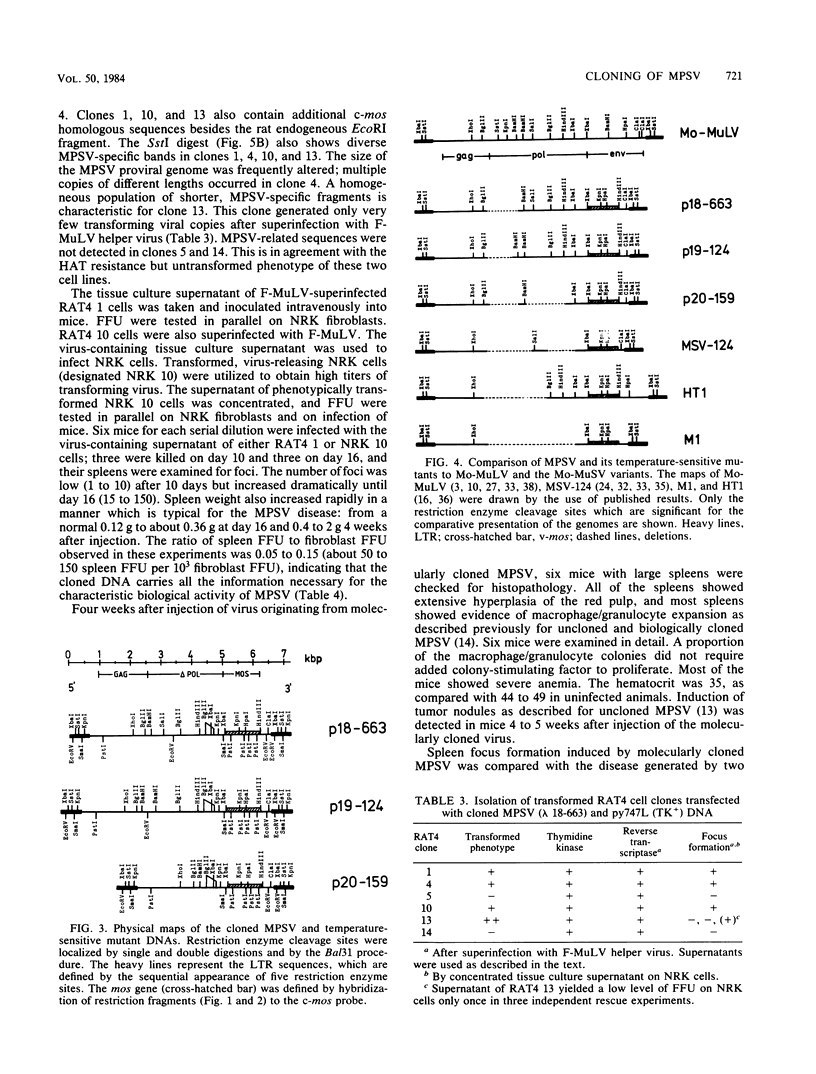
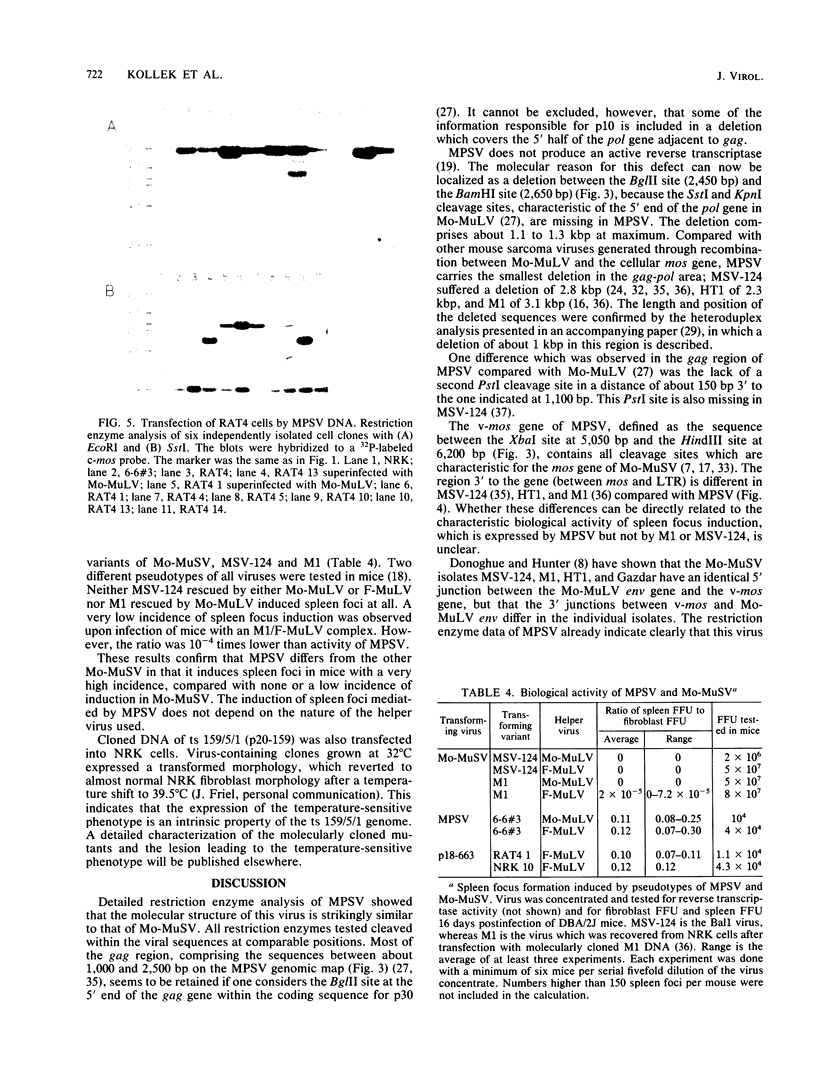


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bassin R. H., Simons P. J., Chesterman F. C., Harvey J. J. Murine sarcoma virus (Harvey): characteristics of focus formation in mouse embryo cell cultures, and virus production by hamster tumor cells. Int J Cancer. 1968 Mar 15;3(2):265–272. doi: 10.1002/ijc.2910030212. [DOI] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Berns A. J., Lai M. H., Bosselman R. A., McKennett M. A., Bacheler L. T., Fan H., Maandag E. C., van der Putten H. V., Verma I. M. Molecular cloning of unintegrated and a portion of integrated moloney murine leukemia viral DNA in bacteriophage lambda. J Virol. 1980 Oct;36(1):254–263. doi: 10.1128/jvi.36.1.254-263.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair D. G., McClements W. L., Oskarsson M. K., Fischinger P. J., Vande Woude G. F. Biological activity of cloned Moloney sarcoma virus DNA: Terminally redundant sequences may enhance transformation efficiency. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3504–3508. doi: 10.1073/pnas.77.6.3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R. L., Horn J. P., Wible L., Arlinghaus R. B., Brinkley B. R. Sequence of events in the transformation process in cells infected with a temperature-sensitive transformation mutant of Moloney murine sarcoma virus. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5593–5597. doi: 10.1073/pnas.78.9.5593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirigos M. A., Scott D., Turner W., Perk K. Biological, pathological and physical characterization of a possible variant of a murine sarcoma virus (Moloney). Int J Cancer. 1968 Mar 15;3(2):223–227. doi: 10.1002/ijc.2910030207. [DOI] [PubMed] [Google Scholar]
- Donoghue D. J. Demonstration of biological activity and nucleotide sequence of an in vitro synthesized clone of the Moloney murine sarcoma virus mos gene. J Virol. 1982 May;42(2):538–546. doi: 10.1128/jvi.42.2.538-546.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue D. J., Hunter T. Recombinational junctions of variants of Moloney murine sarcoma virus: generation and divergence of a mammalian transforming gene. J Virol. 1983 Feb;45(2):607–617. doi: 10.1128/jvi.45.2.607-617.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIEND C. Cell-free transmission in adult Swiss mice of a disease having the character of a leukemia. J Exp Med. 1957 Apr 1;105(4):307–318. doi: 10.1084/jem.105.4.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilboa E., Goff S., Shields A., Yoshimura F., Mitra S., Baltimore D. In vitro synthesis of a 9 kbp terminally redundant DNA carrying the infectivity of Moloney murine leukemia virus. Cell. 1979 Apr;16(4):863–874. doi: 10.1016/0092-8674(79)90101-6. [DOI] [PubMed] [Google Scholar]
- Godson G. N., Vapnek D. A simple method of preparing large amounts of phiX174 RF 1 supercoiled DNA. Biochim Biophys Acta. 1973 Apr 11;299(4):516–520. doi: 10.1016/0005-2787(73)90223-2. [DOI] [PubMed] [Google Scholar]
- Horn J. P., Wood T. G., Blair D. G., Arlinghaus R. B. Partial characterization of a moloney murine sarcoma virus 85,000-dalton polypeptide whose expression correlates with the transformed phenotype in cells infected with a temperature-sensitive mutant virus. Virology. 1980 Sep;105(2):516–525. doi: 10.1016/0042-6822(80)90052-5. [DOI] [PubMed] [Google Scholar]
- Horn J. P., Wood T. G., Murphy E. C., Jr, Blair D. G., Arlinghaus R. B. A selective temperature-sensitive defect in viral RNA expression in cells infected with a ts transformation mutant of murine sarcoma virus. Cell. 1981 Jul;25(1):37–46. doi: 10.1016/0092-8674(81)90229-4. [DOI] [PubMed] [Google Scholar]
- Jähner D., Stuhlmann H., Jaenisch R. Conformation of free and of integrated Moloney leukemia virus proviral DNA in preleukemic and leukemic BALB/Mo mice. Virology. 1980 Feb;101(1):111–123. doi: 10.1016/0042-6822(80)90488-2. [DOI] [PubMed] [Google Scholar]
- Klein B., Le Bousse C., Fagg B., Smajda-Joffe F., Vehmeyer K., Mori K. J., Jasmin C., Ostertag W. Effects of myeloproliferative sarcoma virus on the pluripotential stem cell and granulocyte precursor cell populations of DBA/2 mice. J Natl Cancer Inst. 1981 May;66(5):935–940. [PubMed] [Google Scholar]
- Le Bousse-Kerdiles M. C., Smadja-Joffe F., Klein B., Caillou B., Jasmin C. Study of a virus-induced myeloproliferative syndrome associated with tumor formation in mice. Eur J Cancer. 1980 Jan;16(1):43–51. doi: 10.1016/0014-2964(80)90106-1. [DOI] [PubMed] [Google Scholar]
- McClements W. L., Enquist L. W., Oskarsson M., Sullivan M., Vande Woude G. F. Frequent site-specific deletion of coliphage lambda murine sarcoma virus recombinants and its use in the identification of a retrovirus integration site. J Virol. 1980 Aug;35(2):488–497. doi: 10.1128/jvi.35.2.488-497.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oskarsson M., McClements W. L., Blair D. G., Maizel J. V., Vande Woude G. F. Properties of a normal mouse cell DNA sequence (sarc) homologous to the src sequence of Moloney sarcoma virus. Science. 1980 Mar 14;207(4436):1222–1224. doi: 10.1126/science.6243788. [DOI] [PubMed] [Google Scholar]
- Ostertag W., Vehmeyer K., Fagg B., Pragnell I. B., Paetz W., Le Bousse M. C., Smadja-Joffe F., Klein B., Jasmin C., Eisen H. Myeloproliferative virus, a cloned murine sarcoma virus with spleen focus-forming properties in adult mice. J Virol. 1980 Feb;33(2):573–582. doi: 10.1128/jvi.33.2.573-582.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pragnell I. B., Fusco A., Arbuthnott C., Smadja-Joffe F., Klein B., Jasmin C., Ostertag W. Analysis of the myeloproliferative sarcoma virus genome: limited changes in the prototype lead to altered target cell specificity. J Virol. 1981 Jun;38(3):952–957. doi: 10.1128/jvi.38.3.952-957.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAUSCHER F. J. A virus-induced disease of mice characterized by erythrocytopoiesis and lymphoid leukemia. J Natl Cancer Inst. 1962 Sep;29:515–543. [PubMed] [Google Scholar]
- Reddy E. P., Smith M. J., Aaronson S. A. Complete nucleotide sequence and organization of the Moloney murine sarcoma virus genome. Science. 1981 Oct 23;214(4519):445–450. doi: 10.1126/science.6170110. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Scher C. D., Scolnick E. M., Siegler R. Induction of erythroid leukaemia by Harvey and Kirsten sarcoma viruses. Nature. 1975 Jul 17;256(5514):225–226. doi: 10.1038/256225a0. [DOI] [PubMed] [Google Scholar]
- Shinnick T. M., Lerner R. A., Sutcliffe J. G. Nucleotide sequence of Moloney murine leukaemia virus. Nature. 1981 Oct 15;293(5833):543–548. doi: 10.1038/293543a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stacey A., Arbuthnott C., Kollek R., Coggins L., Ostertag W. Comparison of myeloproliferative sarcoma virus with Moloney murine sarcoma virus variants by nucleotide sequencing and heteroduplex analysis. J Virol. 1984 Jun;50(3):725–732. doi: 10.1128/jvi.50.3.725-732.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steeves R. A. Editorial: Spleen focus-forming virus in Friend and Rauscher leukemia virus preparations. J Natl Cancer Inst. 1975 Feb;54(2):289–297. doi: 10.1093/jnci/54.2.289. [DOI] [PubMed] [Google Scholar]
- Topp W. C. Normal rat cell lines deficient in nuclear thymidine kinase. Virology. 1981 Aug;113(1):408–411. doi: 10.1016/0042-6822(81)90168-9. [DOI] [PubMed] [Google Scholar]
- Tronick S. R., Robbins K. C., Canaani E., Devare S. G., Andersen P. R., Aaronson S. A. Molecular cloning of Moloney murine sarcoma virus: arrangement of virus-related sequences within the normal mouse genome. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6314–6318. doi: 10.1073/pnas.76.12.6314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Beveren C., Galleshaw J. A., Jonas V., Berns A. J., Doolittle R. F., Donoghue D. J., Verma I. M. Nucleotide sequence and formation of the transforming gene of a mouse sarcoma virus. Nature. 1981 Jan 22;289(5795):258–262. doi: 10.1038/289258a0. [DOI] [PubMed] [Google Scholar]
- Van Beveren C., Goddard J. G., Berns A., Verma I. M. Structure of Moloney murine leukemia viral DNA: nucleotide sequence of the 5' long terminal repeat and adjacent cellular sequences. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3307–3311. doi: 10.1073/pnas.77.6.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Beveren C., van Straaten F., Galleshaw J. A., Verma I. M. Nucleotide sequence of the genome of a murine sarcoma virus. Cell. 1981 Nov;27(1 Pt 2):97–108. doi: 10.1016/0092-8674(81)90364-0. [DOI] [PubMed] [Google Scholar]
- Vande Woude G. F., Oskarsson M., Enquist L. W., Nomura S., Sullivan M., Fischinger P. J. Cloning of integrated Moloney sarcoma proviral DNA sequences in bacteriophage lambda. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4464–4468. doi: 10.1073/pnas.76.9.4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma I. M., Lai M. H., Bosselman R. A., McKennett M. A., Fan H., Berns A. Molecular cloning of unintegrated Moloney mouse sarcoma virus DNA in bacteriophage lambda. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1773–1777. doi: 10.1073/pnas.77.4.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma I. M., McKennett M. A. Genome organization of RNA tumor viruses II. Physical maps of in vitro-synthesized Moloney murine leukemia virus double-stranded DNA by restriction endonucleases. J Virol. 1978 Jun;26(3):630–645. doi: 10.1128/jvi.26.3.630-645.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Silverstein S., Lee L. S., Pellicer A., Cheng Y. c., Axel R. Transfer of purified herpes virus thymidine kinase gene to cultured mouse cells. Cell. 1977 May;11(1):223–232. doi: 10.1016/0092-8674(77)90333-6. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. M., Benzinger R., Lawhorne L., Treiber G. Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology. 1970 Mar;40(3):734–744. doi: 10.1016/0042-6822(70)90218-7. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y., Gamble C. L., Clark S. P., Joyner A., Shibuya T., MacDonald M. E., Mager D., Bernstein A., Mak T. W. Clonal analysis of early and late stages of erythroleukemia induced by molecular clones of integrated spleen focus-forming virus. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6893–6897. doi: 10.1073/pnas.78.11.6893. [DOI] [PMC free article] [PubMed] [Google Scholar]