Abstract
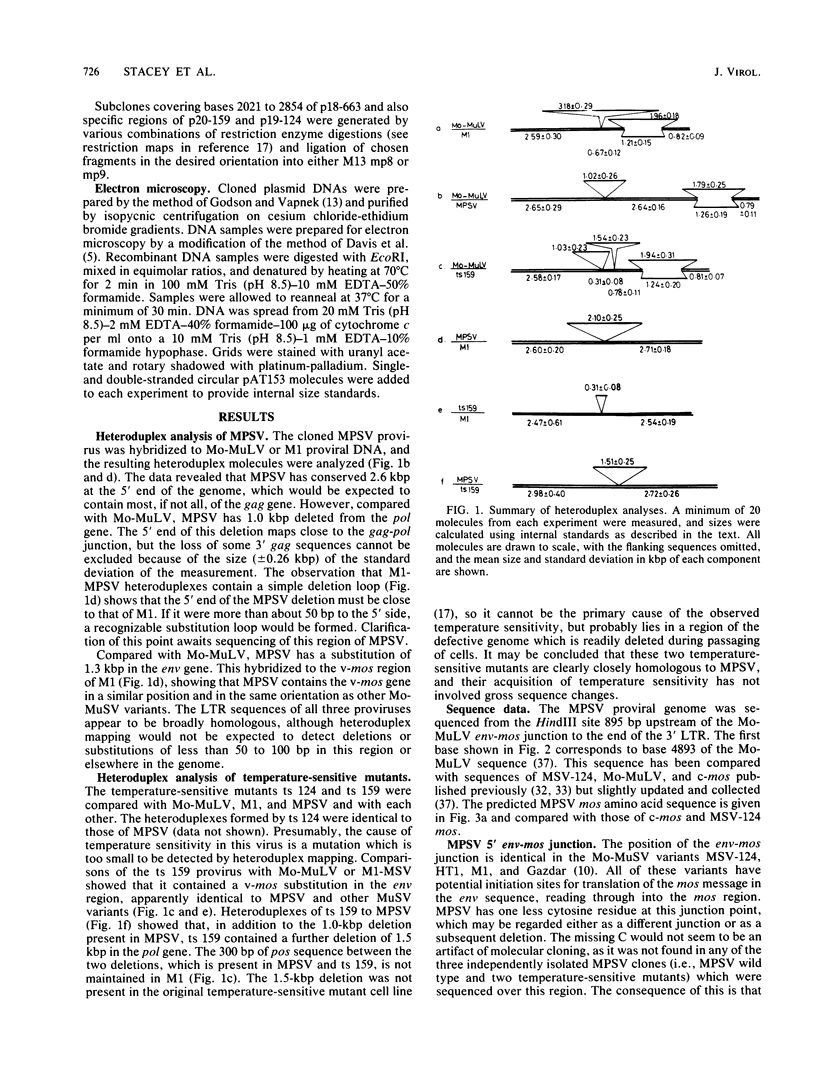
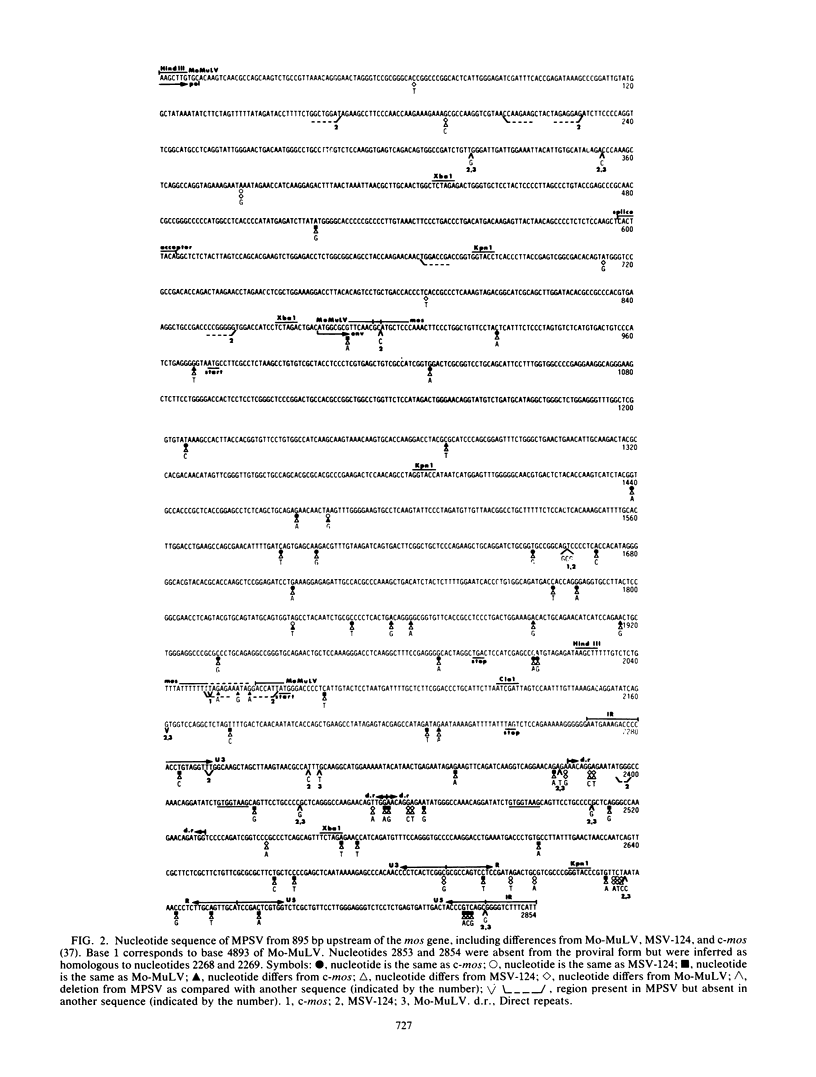
The myeloproliferative sarcoma virus (MPSV) was derived by passage of Moloney sarcoma virus (Mo-MuSV) in adult mice. Mo-MuSV variants transform fibroblasts. However, MPSV also affects erythroid, myeloid, and hematopoietic stem cells. The MPSV proviral genome, two temperature-sensitive mutants derived from it, Mo-MuSV variant M1, and Moloney murine leukemia virus (Mo-MuLV) were compared by heteroduplex mapping. MPSV wild type was found to have 1 kilobase pair deleted from the pol gene and to contain v-mos-related sequences. The 3' end of MPSV, including the oncogene-helper junctions, the v-mos gene, and the 3' long terminal repeat, was sequenced and compared with sequences of Mo-MuLV, MSV-124, and the mouse oncogene c-mos. From these data, MPSV appears to be either closely related to the original Mo-MuSV or an independent recombinant of Mo-MuLV and c-mos. Five possible explanations of the altered specificity of MPSV are considered. (i) The MPSV mos protein has properties inherent in c-mos but lost by other Mo-MuSV mos proteins. (ii) The MPSV mos protein has altered characteristics due to amino acid changes. (iii) Due to a frameshift, MPSV codes for a mos protein truncated at the amino terminal and also a novel peptide. (iv) A second novel peptide may be encoded from the 3' env region. (v) MPSV has long terminal repeats and an enhancer sequence more like Mo-MuLV than Mo-MuSV, with a consequently altered target cell specificity.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S. M., Scolnick E. M. Construction and isolation of a transforming murine retrovirus containing the src gene of Rous sarcoma virus. J Virol. 1983 May;46(2):594–605. doi: 10.1128/jvi.46.2.594-605.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair D. G., Oskarsson M., Wood T. G., McClements W. L., Fischinger P. J., Vande Woude G. G. Activation of the transforming potential of a normal cell sequence: a molecular model for oncogenesis. Science. 1981 May 22;212(4497):941–943. doi: 10.1126/science.7233190. [DOI] [PubMed] [Google Scholar]
- Canaani E., Robbins K. C., Aaronson S. A. The transforming gene of Moloney murine sarcoma virus. Nature. 1979 Nov 22;282(5737):378–383. doi: 10.1038/282378a0. [DOI] [PubMed] [Google Scholar]
- Chirigos M. A., Scott D., Turner W., Perk K. Biological, pathological and physical characterization of a possible variant of a murine sarcoma virus (Moloney). Int J Cancer. 1968 Mar 15;3(2):223–227. doi: 10.1002/ijc.2910030207. [DOI] [PubMed] [Google Scholar]
- DesGroseillers L., Rassart E., Jolicoeur P. Thymotropism of murine leukemia virus is conferred by its long terminal repeat. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4203–4207. doi: 10.1073/pnas.80.14.4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar R., McClements W. L., Enquist L. W., Vande Woude G. F. Nucleotide sequences of integrated Moloney sarcoma provirus long terminal repeats and their host and viral junctions. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3937–3941. doi: 10.1073/pnas.77.7.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue D. J. Demonstration of biological activity and nucleotide sequence of an in vitro synthesized clone of the Moloney murine sarcoma virus mos gene. J Virol. 1982 May;42(2):538–546. doi: 10.1128/jvi.42.2.538-546.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue D. J., Hunter T. Recombinational junctions of variants of Moloney murine sarcoma virus: generation and divergence of a mammalian transforming gene. J Virol. 1983 Feb;45(2):607–617. doi: 10.1128/jvi.45.2.607-617.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue D. J., Sharp P. A., Weinberg R. A. Comparative study of different isolates of murine sarcoma virus. J Virol. 1979 Dec;32(3):1015–1027. doi: 10.1128/jvi.32.3.1015-1027.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H. Transforming genes of retroviruses. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):13–29. doi: 10.1101/sqb.1980.044.01.005. [DOI] [PubMed] [Google Scholar]
- Godson G. N., Vapnek D. A simple method of preparing large amounts of phiX174 RF 1 supercoiled DNA. Biochim Biophys Acta. 1973 Apr 11;299(4):516–520. doi: 10.1016/0005-2787(73)90223-2. [DOI] [PubMed] [Google Scholar]
- Josephs S. F., Dalla-Favera R., Gelmann E. P., Gallo R. C., Wong-Staal F. 5' viral and human cellular sequences corresponding to the transforming gene of simian sarcoma virus. Science. 1983 Feb 4;219(4584):503–505. doi: 10.1126/science.6297002. [DOI] [PubMed] [Google Scholar]
- Katinka M., Vasseur M., Montreau N., Yaniv M., Blangy D. Polyoma DNA sequences involved in control of viral gene expression in murine embryonal carcinoma cells. Nature. 1981 Apr 23;290(5808):720–722. doi: 10.1038/290720a0. [DOI] [PubMed] [Google Scholar]
- Khoury G., Gruss P. Enhancer elements. Cell. 1983 Jun;33(2):313–314. doi: 10.1016/0092-8674(83)90410-5. [DOI] [PubMed] [Google Scholar]
- Kollek R., Stocking C., Smadja-Joffe F., Ostertag W. Molecular cloning and characterization of a leukemia-inducing myeloproliferative sarcoma virus and two of its temperature-sensitive mutants. J Virol. 1984 Jun;50(3):717–724. doi: 10.1128/jvi.50.3.717-724.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laimins L. A., Khoury G., Gorman C., Howard B., Gruss P. Host-specific activation of transcription by tandem repeats from simian virus 40 and Moloney murine sarcoma virus. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6453–6457. doi: 10.1073/pnas.79.21.6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bousse-Kerdiles M. C., Smadja-Joffe F., Klein B., Caillou B., Jasmin C. Study of a virus-induced myeloproliferative syndrome associated with tumor formation in mice. Eur J Cancer. 1980 Jan;16(1):43–51. doi: 10.1016/0014-2964(80)90106-1. [DOI] [PubMed] [Google Scholar]
- Levinson B., Khoury G., Vande Woude G., Gruss P. Activation of SV40 genome by 72-base pair tandem repeats of Moloney sarcoma virus. Nature. 1982 Feb 18;295(5850):568–572. doi: 10.1038/295568a0. [DOI] [PubMed] [Google Scholar]
- Moloney J. B. A virus-induced rhabdomyosarcoma of mice. Natl Cancer Inst Monogr. 1966 Sep;22:139–142. [PubMed] [Google Scholar]
- Ostertag W., Freshney M., Vehmeyer K., Jasmin C., Rutter G. Action of temperature-sensitive mutants of myeloproliferative sarcoma virus suggests that fibroblast-transforming and hematopoietic transforming viral properties are related. J Virol. 1984 Jan;49(1):253–261. doi: 10.1128/jvi.49.1.253-261.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostertag W., Vehmeyer K., Fagg B., Pragnell I. B., Paetz W., Le Bousse M. C., Smadja-Joffe F., Klein B., Jasmin C., Eisen H. Myeloproliferative virus, a cloned murine sarcoma virus with spleen focus-forming properties in adult mice. J Virol. 1980 Feb;33(2):573–582. doi: 10.1128/jvi.33.2.573-582.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papkoff J., Hunter T., Beemon K. In vitro translation of virion RNA from Moloney murine sarcoma virus. Virology. 1980 Feb;101(1):91–103. doi: 10.1016/0042-6822(80)90486-9. [DOI] [PubMed] [Google Scholar]
- Papkoff J., Hunter T. Identification of proteins encoded by the Gazdar murine sarcoma virus genome by in vitro translation and comparison with Moloney murine sarcoma virus 124. J Virol. 1982 Aug;43(2):533–543. doi: 10.1128/jvi.43.2.533-543.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papkoff J., Nigg E. A., Hunter T. The transforming protein of Moloney murine sarcoma virus is a soluble cytoplasmic protein. Cell. 1983 May;33(1):161–172. doi: 10.1016/0092-8674(83)90345-8. [DOI] [PubMed] [Google Scholar]
- Papkoff J., Verma I. M., Hunter T. Detection of a transforming gene product in cells transformed by Moloney murine sarcoma virus. Cell. 1982 Jun;29(2):417–426. doi: 10.1016/0092-8674(82)90158-1. [DOI] [PubMed] [Google Scholar]
- Pragnell I. B., Fusco A., Arbuthnott C., Smadja-Joffe F., Klein B., Jasmin C., Ostertag W. Analysis of the myeloproliferative sarcoma virus genome: limited changes in the prototype lead to altered target cell specificity. J Virol. 1981 Jun;38(3):952–957. doi: 10.1128/jvi.38.3.952-957.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolnick E. M., Howk R. S., Anisowicz A., Peebles P. T., Scher C. D., Parks W. P. Separation of sarcoma virus-specific and leukemia virus-specific genetic sequences of Moloney sarcoma virus. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4650–4654. doi: 10.1073/pnas.72.11.4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinnick T. M., Lerner R. A., Sutcliffe J. G. Nucleotide sequence of Moloney murine leukaemia virus. Nature. 1981 Oct 15;293(5833):543–548. doi: 10.1038/293543a0. [DOI] [PubMed] [Google Scholar]
- Van Beveren C., Galleshaw J. A., Jonas V., Berns A. J., Doolittle R. F., Donoghue D. J., Verma I. M. Nucleotide sequence and formation of the transforming gene of a mouse sarcoma virus. Nature. 1981 Jan 22;289(5795):258–262. doi: 10.1038/289258a0. [DOI] [PubMed] [Google Scholar]
- Vande Woude G. F., Oskarsson M., Enquist L. W., Nomura S., Sullivan M., Fischinger P. J. Cloning of integrated Moloney sarcoma proviral DNA sequences in bacteriophage lambda. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4464–4468. doi: 10.1073/pnas.76.9.4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vande Woude G. F., Oskarsson M., McClements W. L., Enquist L. W., Blair D. G., Fischinger P. J., Maizel J. V., Sullivan M. Characterization of integrated Moloney Sarcoma proviruses and flanking host sequences cloned in bacteriophage lambda. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):735–745. doi: 10.1101/sqb.1980.044.01.079. [DOI] [PubMed] [Google Scholar]
- Weiher H., König M., Gruss P. Multiple point mutations affecting the simian virus 40 enhancer. Science. 1983 Feb 11;219(4585):626–631. doi: 10.1126/science.6297005. [DOI] [PubMed] [Google Scholar]
- Wolff L., Scolnick E., Ruscetti S. Envelope gene of the Friend spleen focus-forming virus: deletion and insertions in 3' gp70/p15E-encoding region have resulted in unique features in the primary structure of its protein product. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4718–4722. doi: 10.1073/pnas.80.15.4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood T. G., McGeady M. L., Blair D. G., Vande Woude G. F. Long terminal repeat enhancement of v-mos transforming activity: identification of essential regions. J Virol. 1983 Jun;46(3):726–736. doi: 10.1128/jvi.46.3.726-736.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Villiers J., Olson L., Tyndall C., Schaffner W. Transcriptional 'enhancers' from SV40 and polyoma virus show a cell type preference. Nucleic Acids Res. 1982 Dec 20;10(24):7965–7976. doi: 10.1093/nar/10.24.7965. [DOI] [PMC free article] [PubMed] [Google Scholar]


