Abstract
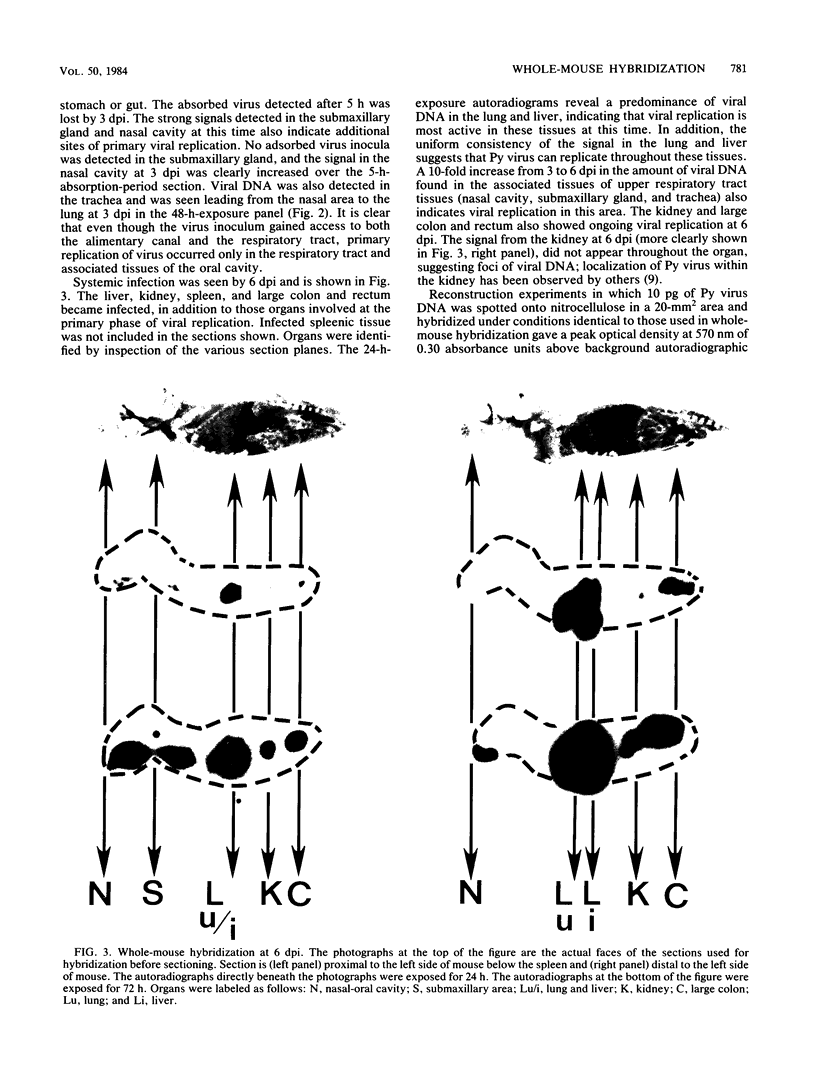
A technique which detects viral DNA or RNA in situ in the organ systems of whole mice is described. Frozen thin sections from whole mice were transferred directly to nitrocellulose and hybridized to labeled viral DNA, allowing the detection of viral DNA or RNA. By this procedure, polyomavirus infection of newborn mice inoculated intranasally was followed. We found that the initial inoculum could be detected in the nasal cavity, lungs, and stomach lining after a 5-h absorption period. Primary replication of virus was observed in the nasal cavity, submaxillary gland, and lungs, followed by a systemic phase of infection in which the liver, spleen, kidney, and large colon also became infected. Viral RNA as well as DNA could also be detected as shown by infecting mice intracerebrally with vesicular stomatitis virus. Vesicular stomatitis virus-specific RNA was observed only in the brains of these mice. It is most likely that this technique can be applied to general molecular studies of mice. With this method we should be able to detect all viruses, bacteria, plasmids, and organ-specific transcripts to which a cloned probe exists.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baringer J. R., Swoveland P. Recovery of herpes-simplex virus from human trigeminal ganglions. N Engl J Med. 1973 Mar 29;288(13):648–650. doi: 10.1056/NEJM197303292881303. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Dubensky T. W., Villarreal L. P. The primary site of replication alters the eventual site of persistent infection by polyomavirus in mice. J Virol. 1984 May;50(2):541–546. doi: 10.1128/jvi.50.2.541-546.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhart W. Complementation and transformation by temperature-sensitive mutants of polyoma virus. Virology. 1969 May;38(1):120–125. doi: 10.1016/0042-6822(69)90133-0. [DOI] [PubMed] [Google Scholar]
- Gallo R. C., Sarin P. S., Gelmann E. P., Robert-Guroff M., Richardson E., Kalyanaraman V. S., Mann D., Sidhu G. D., Stahl R. E., Zolla-Pazner S. Isolation of human T-cell leukemia virus in acquired immune deficiency syndrome (AIDS). Science. 1983 May 20;220(4599):865–867. doi: 10.1126/science.6601823. [DOI] [PubMed] [Google Scholar]
- Goldberg D. A. Isolation and partial characterization of the Drosophila alcohol dehydrogenase gene. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5794–5798. doi: 10.1073/pnas.77.10.5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heritage J., Chesters P. M., McCance D. J. The persistence of papovavirus BK DNA sequences in normal human renal tissue. J Med Virol. 1981;8(2):143–150. doi: 10.1002/jmv.1890080208. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- LEVINTHAL J. D., JAKOBOVITS M., EATON M. D. Polyoma disease and tumors in mice: the distribution of viral antigen detected by immunofluorescence. Virology. 1962 Mar;16:314–319. doi: 10.1016/0042-6822(62)90252-0. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- McCance D. J. Growth and persistence of polyoma early region deletion mutants in mice. J Virol. 1981 Sep;39(3):958–962. doi: 10.1128/jvi.39.3.958-962.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCance D. J., Mims C. A. Reactivation of polyoma virus in kidneys of persistently infected mice during pregnancy. Infect Immun. 1979 Sep;25(3):998–1002. doi: 10.1128/iai.25.3.998-1002.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCance D. J., Mims C. A. Transplacental transmission of polyoma virus in mice. Infect Immun. 1977 Oct;18(1):196–202. doi: 10.1128/iai.18.1.196-202.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. R., Barrett R. E., Britton C. B., Tapper M. L., Bahr G. S., Bruno P. J., Marquardt M. D., Hays A. P., McMurtry J. G., 3rd, Weissman J. B. Progressive multifocal leukoencephalopathy in a male homosexual with T-cell immune deficiency. N Engl J Med. 1982 Dec 2;307(23):1436–1438. doi: 10.1056/NEJM198212023072307. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Buchmeier M. J. Restricted expression of viral glycoprotein in cells of persistently infected mice. Nature. 1982 Nov 25;300(5890):360–362. doi: 10.1038/300360a0. [DOI] [PubMed] [Google Scholar]
- ROSEN L., HOVIS J. F., BELL J. A. Further observation on typing adenoviruses and a description of two possible additional serotypes. Proc Soc Exp Biol Med. 1962 Aug-Sep;110:710–713. doi: 10.3181/00379727-110-27626. [DOI] [PubMed] [Google Scholar]
- ROWE W. P., HARTLEY J. W., ESTES J. D., HUEBNER R. J. Growth curves of polyoma virus in mice and hamsters. Natl Cancer Inst Monogr. 1960 Sep;4:189–209. [PubMed] [Google Scholar]
- ROWE W. P., HARTLEY J. W., ESTES J. D., HUEBNER R. J. Studies of mouse polyoma virus infection. 1. Procedures for quantitation and detection of virus. J Exp Med. 1959 Apr 1;109(4):379–391. doi: 10.1084/jem.109.4.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Schrier R. D., Pizer L. I., Moorhead J. W. Tolerance and suppression of immunity to herpes simplex virus: different presentations of antigens induce different types of suppressor cells. Infect Immun. 1983 May;40(2):514–522. doi: 10.1128/iai.40.2.514-522.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Villarreal L. P., Berg P. Hybridization in situ of SV40 plaques: detection of recombinant SV40 virus carrying specific sequences of nonviral DNA. Science. 1977 Apr 8;196(4286):183–185. doi: 10.1126/science.191907. [DOI] [PubMed] [Google Scholar]






