Abstract
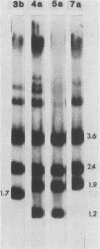
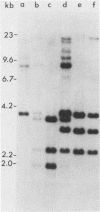
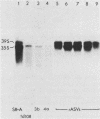
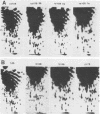
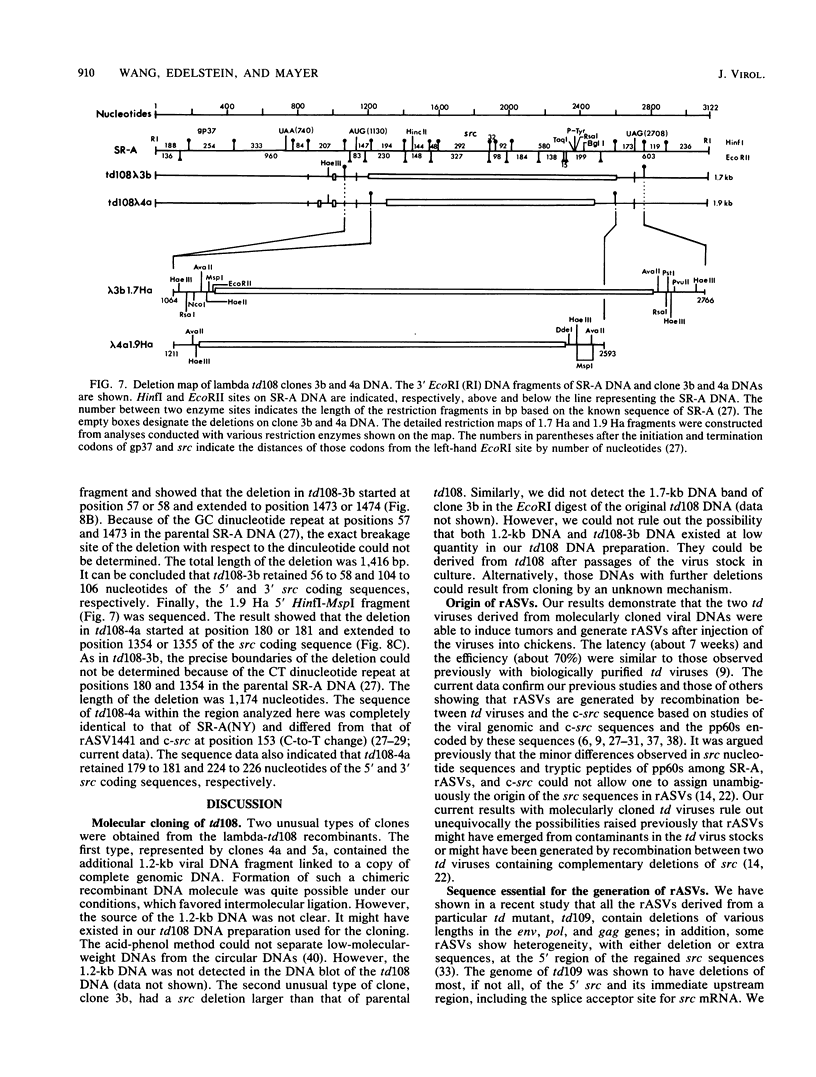
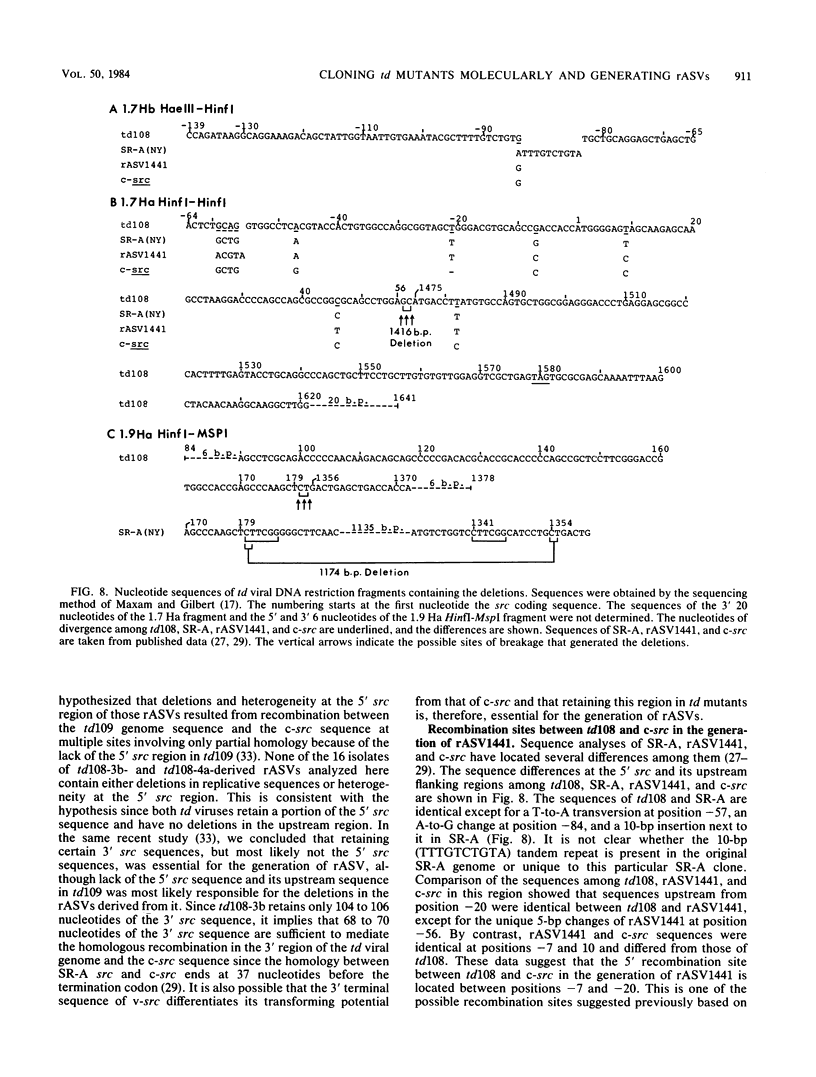
td108 , a transformation-defective (td) deletion mutant of the Schmidt-Ruppin strain of Rous sarcoma virus of subgroup A (SR-A), was molecularly cloned. Two isolates of td viruses, td108 -3b and td108 -4a, obtained by transfection of the molecularly cloned td108 DNAs into chicken embryo fibroblasts, were tested for their ability to induce tumors and generate recovered avian sarcoma viruses ( rASVs ) in chickens. Both td viruses were able to induce tumors with a latency and frequency similar to those observed previously with biologically purified td mutants of SR-A. rASVs were isolated from most of the tumors examined. The genomic RNAs of those newly obtained rASVs were analyzed by RNase T1 oligonucleotide fingerprinting. The results showed that they had regained the deleted src sequences and contained the same set of marker src oligonucleotides as those of rASVs analyzed previously. The src oligonucleotides of rASVs are distinguishable from those present in SR-A. We conclude that those rASVs must have been generated by recombination between the molecularly cloned td mutants and the c-src sequence. The deletions in the td mutants were mapped by restriction enzyme analysis and nucleotide sequencing. td108 -3b was found to contain an internal src deletion of 1,416 nucleotides and to retain 57 and 105 nucleotides of the 5' and 3' src coding sequences, respectively. td108 -4a contained a src deletion of 1,174 nucleotides and retained 180 and 225 nucleotides of the 5' and 3' src sequences, respectively. Comparison of sequences in the 5' src and its upstream region of td108 -3b with those of SR-A, rASV1441 (a td108 -derived rASV analyzed previously), and c-src suggested that the 5' recombination between td108 and c-src occurred from 7 to 20 nucleotides upstream from the beginning of the src coding sequence.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Blattner F. R., Blechl A. E., Denniston-Thompson K., Faber H. E., Richards J. E., Slightom J. L., Tucker P. W., Smithies O. Cloning human fetal gamma globin and mouse alpha-type globin DNA: preparation and screening of shotgun collections. Science. 1978 Dec 22;202(4374):1279–1284. doi: 10.1126/science.725603. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Czernilofsky A. P., Levinson A. D., Varmus H. E., Bishop J. M., Tischer E., Goodman H. M. Nucleotide sequence of an avian sarcoma virus oncogene (src) and proposed amino acid sequence for gene product. Nature. 1980 Sep 18;287(5779):198–203. doi: 10.1038/287198a0. [DOI] [PubMed] [Google Scholar]
- DeLorbe W. J., Luciw P. A., Goodman H. M., Varmus H. E., Bishop J. M. Molecular cloning and characterization of avian sarcoma virus circular DNA molecules. J Virol. 1980 Oct;36(1):50–61. doi: 10.1128/jvi.36.1.50-61.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enrietto P. J., Payne L. N., Wyke J. A. Analysis of the pathogenicity of transformation defective partial deletion mutants of avian sarcoma virus: characterization of recovered viruses which encode novel src specific proteins. Virology. 1983 Jun;127(2):397–411. doi: 10.1016/0042-6822(83)90153-8. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Hanafusa H., Halpern C. C., Buchhagen D. L., Kawai S. Recovery of avian sarcoma virus from tumors induced by transformation-defective mutants. J Exp Med. 1977 Dec 1;146(6):1735–1747. doi: 10.1084/jem.146.6.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanafusa H. Rapid transformation of cells by Rous sarcoma virus. Proc Natl Acad Sci U S A. 1969 Jun;63(2):318–325. doi: 10.1073/pnas.63.2.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Karess R. E., Hanafusa H. Viral and cellular src genes contribute to the structure of recovered avian sarcoma virus transforming protein. Cell. 1981 Apr;24(1):155–164. doi: 10.1016/0092-8674(81)90511-0. [DOI] [PubMed] [Google Scholar]
- Kawai S., Duesberg P. H., Hanafusa H. Transformation-defective mutants of Rous sarcoma virus with src gene deletions of varying length. J Virol. 1977 Dec;24(3):910–914. doi: 10.1128/jvi.24.3.910-914.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai S., Hanafusa H. Genetic recombination with avian tumor virus. Virology. 1972 Jul;49(1):37–44. doi: 10.1016/s0042-6822(72)80005-9. [DOI] [PubMed] [Google Scholar]
- Lee W. H., Nunn M., Duesberg P. H. src Genes of ten Rous sarcoma virus strains, including two reportedly transduced from the cell, are completely allelic; putative markers of transduction are not detected. J Virol. 1981 Sep;39(3):758–776. doi: 10.1128/jvi.39.3.758-776.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G. S., Duesberg P. H. The a subunit in the RNA of transforming avian tumor viruses. I. Occurrence in different virus strains. II. Spontaneous loss resulting in nontransforming variants. Virology. 1972 Feb;47(2):494–497. doi: 10.1016/0042-6822(72)90287-5. [DOI] [PubMed] [Google Scholar]
- Mason W. S., Hsu T. W., Yeater C., Sabran J. L., Mark G. E., Kaji A., Taylor J. M. Avian sarcoma virus-transformed quail clones defective in the production of focus-forming virus. J Virol. 1979 Apr;30(1):132–140. doi: 10.1128/jvi.30.1.132-140.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscovici C., Moscovici M. G., Jimenez H., Lai M. M., Hayman M. J., Vogt P. K. Continuous tissue culture cell lines derived from chemically induced tumors of Japanese quail. Cell. 1977 May;11(1):95–103. doi: 10.1016/0092-8674(77)90320-8. [DOI] [PubMed] [Google Scholar]
- Murray N. E., Brammar W. J., Murray K. Lambdoid phages that simplify the recovery of in vitro recombinants. Mol Gen Genet. 1977 Jan 7;150(1):53–61. doi: 10.1007/BF02425325. [DOI] [PubMed] [Google Scholar]
- Omer C. A., Pogue-Geile K., Guntaka R., Staskus K. A., Faras A. J. Involvement of directly repeated sequences in the generation of deletions of the avian sarcoma virus src gene. J Virol. 1983 Aug;47(2):380–382. doi: 10.1128/jvi.47.2.380-382.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins T., Duesberg P. Specific RNA sequences of Rous sarcoma virus (RSV) recovered from tumors induced by transformation-defective RSV deletion mutants. Virology. 1979 Mar;93(2):427–434. doi: 10.1016/0042-6822(79)90246-0. [DOI] [PubMed] [Google Scholar]
- Rubin C. M., Schmid C. W. Pyrimidine-specific chemical reactions useful for DNA sequencing. Nucleic Acids Res. 1980 Oct 24;8(20):4613–4619. doi: 10.1093/nar/8.20.4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz D. E., Tizard R., Gilbert W. Nucleotide sequence of Rous sarcoma virus. Cell. 1983 Mar;32(3):853–869. doi: 10.1016/0092-8674(83)90071-5. [DOI] [PubMed] [Google Scholar]
- Shibuya M., Wang L. H., Hanafusa H. Molecular cloning of the Fujinami sarcoma virus genome and its comparison with sequences of other related transforming viruses. J Virol. 1982 Jun;42(3):1007–1016. doi: 10.1128/jvi.42.3.1007-1016.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Takeya T., Feldman R. A., Hanafusa H. DNA sequence of the viral and cellular src gene of chickens. 1. Complete nucleotide sequence of an EcoRI fragment of recovered avian sarcoma virus which codes for gp37 and pp60src. J Virol. 1982 Oct;44(1):1–11. doi: 10.1128/jvi.44.1.1-11.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeya T., Hanafusa H. DNA sequence of the viral and cellular src gene of chickens. II. Comparison of the src genes of two strains of avian sarcoma virus and of the cellular homolog. J Virol. 1982 Oct;44(1):12–18. doi: 10.1128/jvi.44.1.12-18.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeya T., Hanafusa H. Structure and sequence of the cellular gene homologous to the RSV src gene and the mechanism for generating the transforming virus. Cell. 1983 Mar;32(3):881–890. doi: 10.1016/0092-8674(83)90073-9. [DOI] [PubMed] [Google Scholar]
- Vigne R., Breitman M. L., Moscovici C., Vogt P. K. Restitution of fibroblast-transforming ability in src deletion mutants of avian sarcoma virus during animal passage. Virology. 1979 Mar;93(2):413–426. doi: 10.1016/0042-6822(79)90245-9. [DOI] [PubMed] [Google Scholar]
- Vigne R., Neil J. C., Breitman M. L., Vogt P. K. Recovered src genes are polymorphic and contain host markers. Virology. 1980 Aug;105(1):71–85. doi: 10.1016/0042-6822(80)90157-9. [DOI] [PubMed] [Google Scholar]
- Vogt P. K. Spontaneous segregation of nontransforming viruses from cloned sarcoma viruses. Virology. 1971 Dec;46(3):939–946. doi: 10.1016/0042-6822(71)90092-4. [DOI] [PubMed] [Google Scholar]
- Wang L. H., Beckson M., Anderson S. M., Hanafusa H. Identification of the viral sequence required for the generation of recovered avian sarcoma viruses and characterization of a series of replication-defective recovered avian sarcoma viruses. J Virol. 1984 Mar;49(3):881–891. doi: 10.1128/jvi.49.3.881-891.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. H., Duesberg P., Beemon K., Vogt P. K. Mapping RNase T1-resistant oligonucleotides of avian tumor virus RNAs: sarcoma-specific oligonucleotides are near the poly(A) end and oligonucleotides common to sarcoma and transformation-defective viruses are at the poly(A) end. J Virol. 1975 Oct;16(4):1051–1070. doi: 10.1128/jvi.16.4.1051-1070.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. H., Duesberg P. Properties and location of poly(A) in Rous sarcoma virus RNA. J Virol. 1974 Dec;14(6):1515–1529. doi: 10.1128/jvi.14.6.1515-1529.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. H., Feldman R., Shibuya M., Hanafusa H., Notter M. F., Balduzzi P. C. Genetic structure, transforming sequence, and gene product of avian sarcoma virus UR1. J Virol. 1981 Oct;40(1):258–267. doi: 10.1128/jvi.40.1.258-267.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. H., Halpern C. C., Nadel M., Hanafusa H. Recombination between viral and cellular sequences generates transforming sarcoma virus. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5812–5816. doi: 10.1073/pnas.75.12.5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. H., Moscovici C., Karess R. E., Hanafusa H. Analysis of the src gene of sarcoma viruses generated by recombination between transformation-defective mutants and quail cellular sequences. J Virol. 1979 Nov;32(2):546–556. doi: 10.1128/jvi.32.2.546-556.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M., Yamashita M., Nomoto A. Transformation-defective mutants of Rous sarcoma virus with longer sizes of genome RNA and their highly frequent occurrences. J Virol. 1979 May;30(2):453–461. doi: 10.1128/jvi.30.2.453-461.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zasloff M., Ginder G. D., Felsenfeld G. A new method for the purification and identification of covalently closed circular DNA molcules. Nucleic Acids Res. 1978 Apr;5(4):1139–1152. doi: 10.1093/nar/5.4.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]