Abstract
BXH-2 recombinant inbred (RI) mice produce high titers of B-ecotropic murine leukemia virus beginning early in life and have a high incidence of non-T-cell leukemias that occur before 1 year of age. The leukemias that develop are in some cases associated with hind limb paralysis. In addition, a dualtropic mink cell focus-forming virus has been isolated from leukemic cells of BXH-2 mice. Immunological and cytochemical characterization of the BXH-2 leukemias showed that they are of the myeloid lineage. To assess the oncogenicity of the BXH-2 viruses, newborn mice of several BXH RI strains were inoculated at birth with biologically cloned B-ecotropic or mink cell focus-forming murine leukemia virus. These studies demonstrated that the B-ecotropic virus can induce myeloid leukemias in other BXH RI strains, whereas the dualtropic mink cell focus-forming isolates were nononcogenic in the strains tested. DNA-DNA reassociation analysis indicated that the organotropism of the B-ecotropic murine leukemia virus is confined to lymphoid tissues. Southern analysis of tumor DNAs showed that there was amplification of ecotropic virus-specific sequences in BXH-2 myeloid tumors and in all leukemias induced in other BXH RI strains by inoculation of the BXH-2 B-ecotropic virus. Although B-ecotropic virus is expressed in central nervous tissues of paralyzed BXH-2 mice, we were unable to induce the disorder in several BXH RI strains inoculated intracranially at birth with either the B-ecotropic or dualtropic virus. These results suggest that the paralysis that occurs in BXH-2 mice is due to the infiltration of leukemic cells into the central nervous system.
Full text
PDF


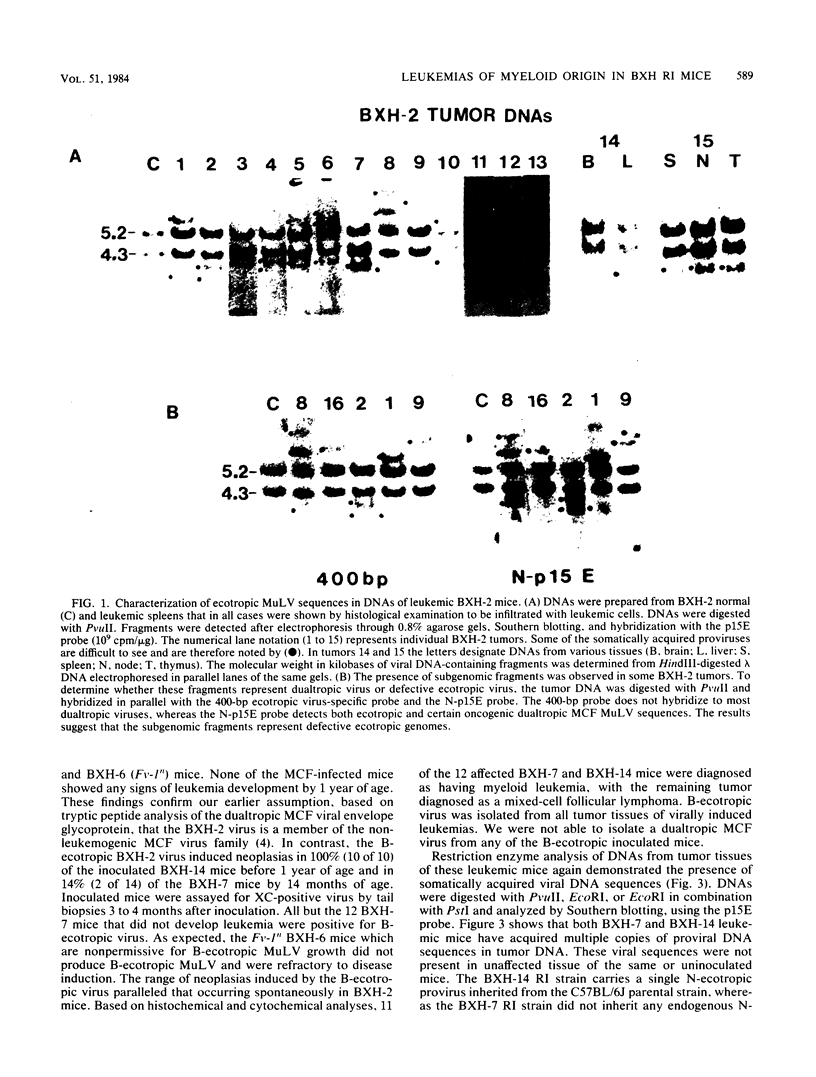

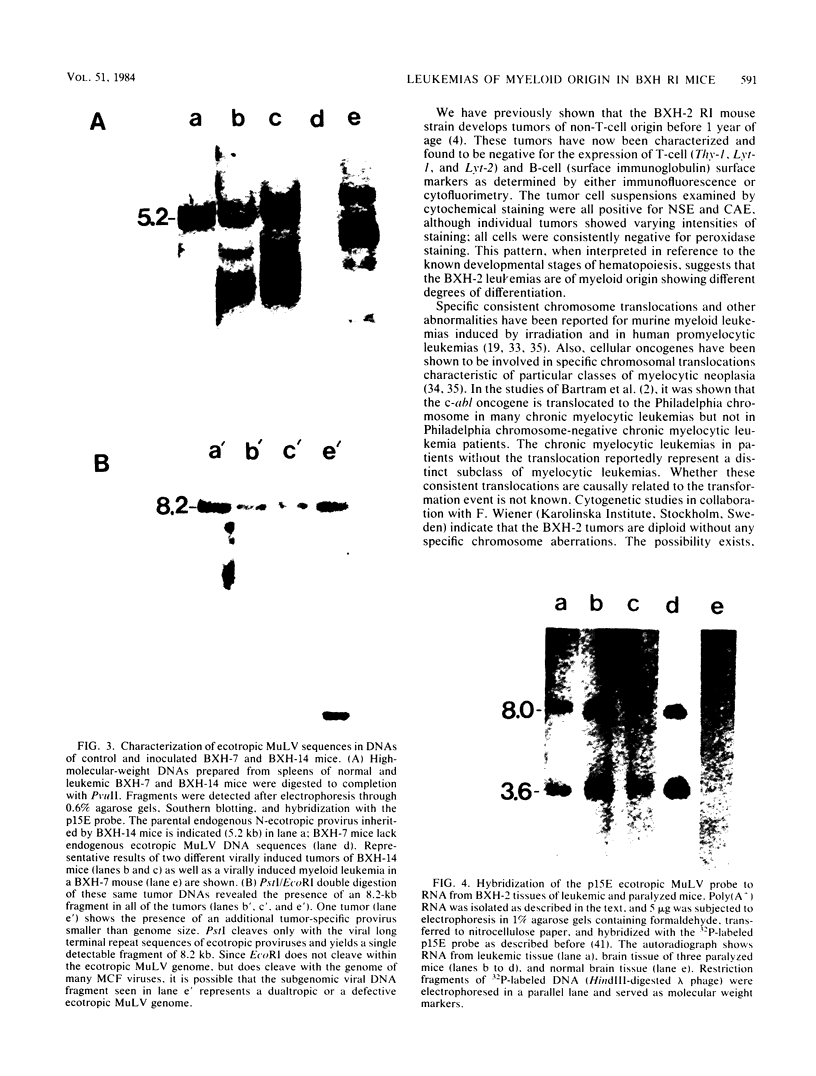



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asjö B., Fenyö E. M., Klein G. Moloney virus (M-MuLV) leukemogenesis: virus spread, antibody production and antigenic expression in neonatally virus-inoculated young mice. Int J Cancer. 1981 Jul 15;28(1):65–70. doi: 10.1002/ijc.2910280112. [DOI] [PubMed] [Google Scholar]
- Bartram C. R., de Klein A., Hagemeijer A., van Agthoven T., Geurts van Kessel A., Bootsma D., Grosveld G., Ferguson-Smith M. A., Davies T., Stone M. Translocation of c-ab1 oncogene correlates with the presence of a Philadelphia chromosome in chronic myelocytic leukaemia. Nature. 1983 Nov 17;306(5940):277–280. doi: 10.1038/306277a0. [DOI] [PubMed] [Google Scholar]
- Bedigian H. G., Copeland N. G., Jenkins N. A., Salvatore K., Rodick S. Emv-13 (Akv-3): a noninducible endogenous ecotropic provirus of AKR/J mice. J Virol. 1983 May;46(2):490–497. doi: 10.1128/jvi.46.2.490-497.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedigian H. G., Taylor B. A., Meier H. Expression of murine leukemia viruses in the highly lymphomatous BXH-2 recombinant inbred mouse strain. J Virol. 1981 Aug;39(2):632–640. doi: 10.1128/jvi.39.2.632-640.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benade L. E., Ihle J. N., Declève A. Serological characterization of B-tropic viruses of C57BL mice: possible origin by recombination of endogenous N-tropic and xenotropic viruses. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4553–4557. doi: 10.1073/pnas.75.9.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavenee W. K., Dryja T. P., Phillips R. A., Benedict W. F., Godbout R., Gallie B. L., Murphree A. L., Strong L. C., White R. L. Expression of recessive alleles by chromosomal mechanisms in retinoblastoma. 1983 Oct 27-Nov 2Nature. 305(5937):779–784. doi: 10.1038/305779a0. [DOI] [PubMed] [Google Scholar]
- Chatis P. A., Holland C. A., Hartley J. W., Rowe W. P., Hopkins N. Role for the 3' end of the genome in determining disease specificity of Friend and Moloney murine leukemia viruses. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4408–4411. doi: 10.1073/pnas.80.14.4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Cloyd M. W., Linemeyer D. L., Lander M. R., Rands E., Lowy D. R. Cellular origin and role of mink cell focus-forming viruses in murine thymic lymphomas. Nature. 1982 Jan 7;295(5844):25–31. doi: 10.1038/295025a0. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Lander M. R., Rands E., Lowy D. R. Structure of endogenous murine leukemia virus DNA in mouse genomes. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5774–5778. doi: 10.1073/pnas.77.10.5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesebro B., Portis J. L., Wehrly K., Nishio J. Effect of murine host genotype on MCF virus expression, latency, and leukemia cell type of leukemias induced by Friend murine leukemia helper virus. Virology. 1983 Jul 15;128(1):221–233. doi: 10.1016/0042-6822(83)90332-x. [DOI] [PubMed] [Google Scholar]
- Cloyd M. W., Hartley J. W., Rowe W. P. Genetic study of lymphoma induction by AKR mink cell focus-inducing virus in AKR x NFS crosses. J Exp Med. 1981 Aug 1;154(2):450–457. doi: 10.1084/jem.154.2.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloyd M. W., Hartley J. W., Rowe W. P. Lymphomagenicity of recombinant mink cell focus-inducing murine leukemia viruses. J Exp Med. 1980 Mar 1;151(3):542–552. doi: 10.1084/jem.151.3.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DesGroseillers L., Rassart E., Jolicoeur P. Thymotropism of murine leukemia virus is conferred by its long terminal repeat. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4203–4207. doi: 10.1073/pnas.80.14.4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner M. B., Rasheed S. Retroviruses in feral mice. Int Rev Exp Pathol. 1982;23:209–267. [PubMed] [Google Scholar]
- Gautsch J. W., Elder J. H., Jensen F. C., Lerner R. A. In vitro construction of a B-tropic virus by recombination: B-tropism is a cryptic phenotype of xenotropic murine retroviruses. Proc Natl Acad Sci U S A. 1980 May;77(5):2989–2993. doi: 10.1073/pnas.77.5.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazit A., Yaniv A., Ilani A., Ianconescu M., Perk K., Zimber A. Genetic control of the organ specificity of lymphoproliferative disease virus (LPDV) of turkeys. Int J Cancer. 1983 Mar 15;31(3):351–356. doi: 10.1002/ijc.2910310316. [DOI] [PubMed] [Google Scholar]
- Hartley J. W., Yetter R. A., Morse H. C., 3rd A mouse gene on chromosome 5 that restricts infectivity of mink cell focus-forming recombinant murine leukemia viruses. J Exp Med. 1983 Jul 1;158(1):16–24. doi: 10.1084/jem.158.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayata I., Seki M., Yoshida K., Hirashima K., Sado T., Yamagiwa J., Ishihara T. Chromosomal aberrations observed in 52 mouse myeloid leukemias. Cancer Res. 1983 Jan;43(1):367–373. [PubMed] [Google Scholar]
- Herr W., Gilbert W. Somatically acquired recombinant murine leukemia proviruses in thymic leukemias of AKR/J mice. J Virol. 1983 Apr;46(1):70–82. doi: 10.1128/jvi.46.1.70-82.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENKINS V. K., UPTON A. C. CELL-FREE TRANSMISSION OF RADIOGENIC MYELOID LEUKEMIA IN THE MOUSE. Cancer Res. 1963 Dec;23:1748–1755. [PubMed] [Google Scholar]
- Jaenisch R., Mintz B. Simian virus 40 DNA sequences in DNA of healthy adult mice derived from preimplantation blastocysts injected with viral DNA. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1250–1254. doi: 10.1073/pnas.71.4.1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenisch R. Moloney leukemia virus gene expression and gene amplification in preleukemic and leukemic BALB/Mo mice. Virology. 1979 Feb;93(1):80–90. doi: 10.1016/0042-6822(79)90277-0. [DOI] [PubMed] [Google Scholar]
- Jenkins N. A., Copeland N. G., Taylor B. A., Bedigian H. G., Lee B. K. Ecotropic murine leukemia virus DNA content of normal and lymphomatous tissues of BXH-2 recombinant inbred mice. J Virol. 1982 May;42(2):379–388. doi: 10.1128/jvi.42.2.379-388.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanier L. L., Warner N. L., Ledbetter J. A., Herzenberg L. A. Quantitative immunofluorescent analysis of surface phenotypes of murine B cell lymphomas and plasmacytomas with monoclonal antibodies. J Immunol. 1981 Oct;127(4):1691–1697. [PubMed] [Google Scholar]
- Lee J. C., Ihle J. N. Mechanisms of C-type viral leukemogenesis. I. Correlation of in vitro lymphocyte blastogenesis to viremia and leukemia. J Immunol. 1979 Nov;123(5):2351–2358. [PubMed] [Google Scholar]
- Nowinski R. C., Hays E. F. Oncogenicity of AKR endogenous leukemia viruses. J Virol. 1978 Jul;27(1):13–18. doi: 10.1128/jvi.27.1.13-18.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusse R., Varmus H. E. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell. 1982 Nov;31(1):99–109. doi: 10.1016/0092-8674(82)90409-3. [DOI] [PubMed] [Google Scholar]
- Peters R. L., Spahn G. J., Rabstein L. S., Kelloff G. J., Huebner R. J. Neoplasm induction by murine type-C viruses passaged directly from spontaneous non-lymphoreticular tumours. Nat New Biol. 1973 Jul 25;244(134):103–105. doi: 10.1038/newbio244103a0. [DOI] [PubMed] [Google Scholar]
- Prywes R., Foulkes J. G., Rosenberg N., Baltimore D. Sequences of the A-MuLV protein needed for fibroblast and lymphoid cell transformation. Cell. 1983 Sep;34(2):569–579. doi: 10.1016/0092-8674(83)90389-6. [DOI] [PubMed] [Google Scholar]
- Rands E., Lowy D. R., Lander M. R., Chattopadhyay S. K. Restriction endonuclease mapping of ecotropic murine leukemia viral DNAs: size and sequence heterogeneity of the long terminal repeat. Virology. 1981 Jan 30;108(2):445–452. doi: 10.1016/0042-6822(81)90451-7. [DOI] [PubMed] [Google Scholar]
- Rassart E., Sankar-Mistry P., Lemay G., DesGroseillers L., Jolicoeur P. New class of leukemogenic ecotropic recombinant murine leukemia virus isolated from radiation-induced thymomas of C57BL/6 mice. J Virol. 1983 Feb;45(2):565–575. doi: 10.1128/jvi.45.2.565-575.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley J. D. Identification of the constant chromosome regions involved in human hematologic malignant disease. Science. 1982 May 14;216(4547):749–751. doi: 10.1126/science.7079737. [DOI] [PubMed] [Google Scholar]
- Sheer D., Hiorns L. R., Stanley K. F., Goodfellow P. N., Swallow D. M., Povey S., Heisterkamp N., Groffen J., Stephenson J. R., Solomon E. Genetic analysis of the 15;17 chromosome translocation associated with acute promyelocytic leukemia. Proc Natl Acad Sci U S A. 1983 Aug;80(16):5007–5011. doi: 10.1073/pnas.80.16.5007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver J. E., Fredrickson T. N. A new gene that controls the type of leukemia induced by Friend murine leukemia virus. J Exp Med. 1983 Aug 1;158(2):493–505. doi: 10.1084/jem.158.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steeves R. A., Eckner R. J., Bennett M., Mirand E. A., Trudel P. J. Isolation and characterization of a lymphatic leukemia virus in the Friend virus complex. J Natl Cancer Inst. 1971 Jun;46(6):1209–1217. [PubMed] [Google Scholar]
- Tanaka T., Craig A. W. Cell-free transmission of murine myeloid leukaemia. Eur J Cancer. 1970 Aug;6(4):329–333. doi: 10.1016/0014-2964(70)90098-8. [DOI] [PubMed] [Google Scholar]
- Tennant R. W., Otten J. A., Wang T. W., Liou R. S., Brown A., Yang W. K. Control of RFM strain endogenous retrovirus in RFM mouse cells. J Virol. 1983 Jan;45(1):47–54. doi: 10.1128/jvi.45.1.47-54.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upton A. C., Jenkins V. K., Walburg H. E., Jr, Tyndall R. L., Conklin J. W., Wald N. Observations on viral, chemical, and radiation-induced myeloid and lymphoid leukemias in RF mice. Natl Cancer Inst Monogr. 1966 Sep;22:329–347. [PubMed] [Google Scholar]
- Vogt M. Virus cloned from the Rauscher virus complex induces erythroblastosis and thymic lymphoma. Virology. 1982 Apr 15;118(1):225–228. doi: 10.1016/0042-6822(82)90336-1. [DOI] [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Crosby W. H. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971 Mar;55(3):283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]