Abstract
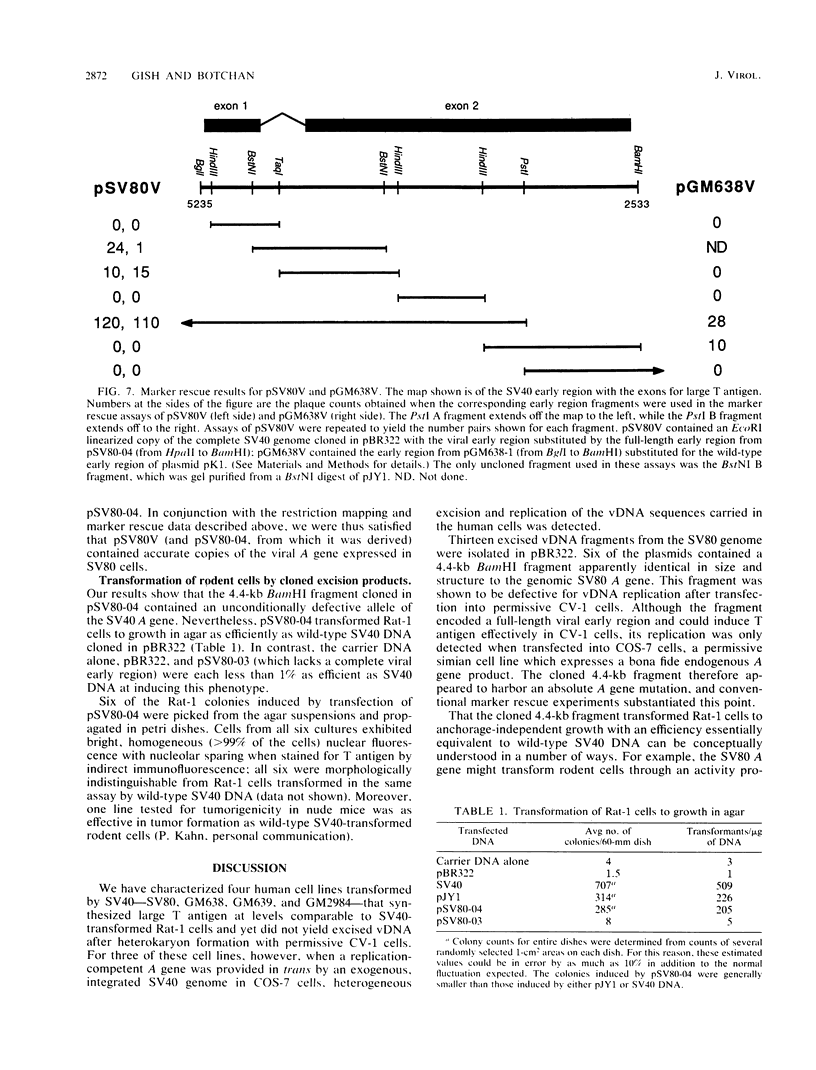
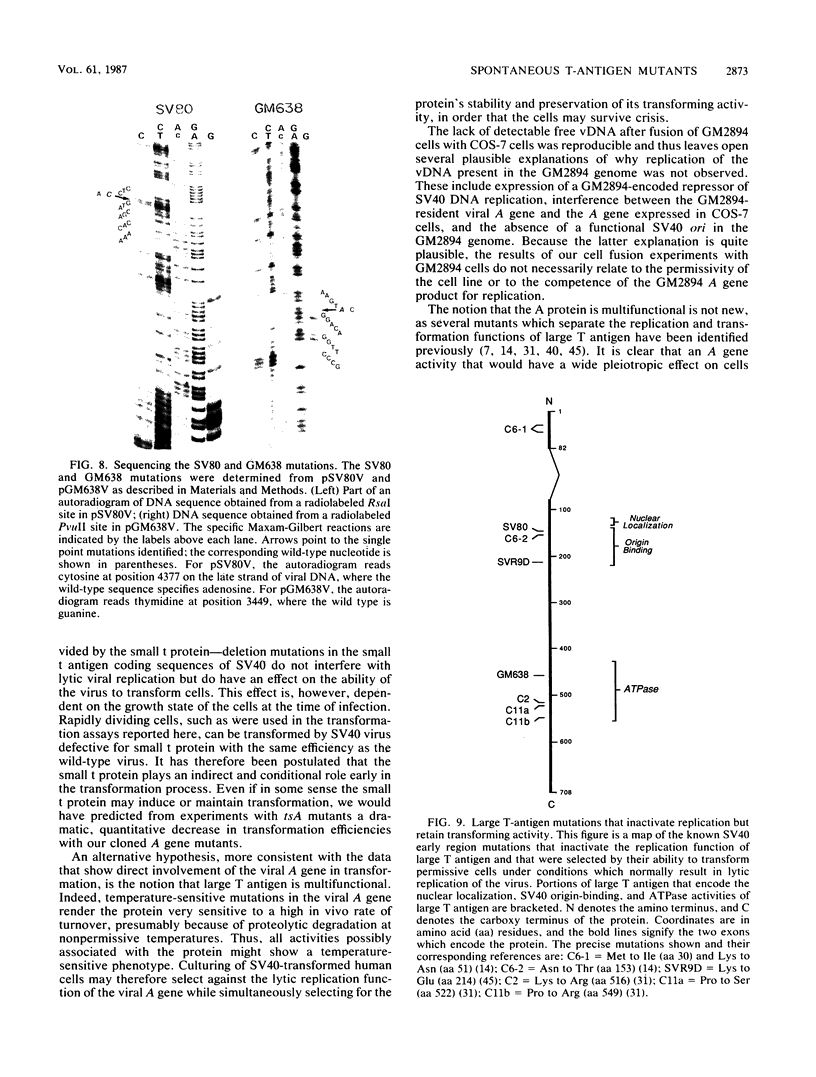
Many types of human cells cultured in vitro are generally semipermissive for simian virus 40 (SV40) replication. Consequently, subpopulations of stably transformed human cells often carry free viral DNA, which is presumed to arise via spontaneous excision from an integrated DNA template. Stably transformed human cell lines that do not have detectable free DNA are therefore likely to harbor harbor mutant viral genomes incapable of excision and replication, or these cells may synthesize variant cellular proteins necessary for viral replication. We examined four such cell lines and conclude that for the three lines SV80, GM638, and GM639, the cells did indeed harbor spontaneous T-antigen mutants. For the SV80 line, marker rescue (determined by a plaque assay) and DNA sequence analysis of cloned DNA showed that a single point mutation converting serine 147 to asparagine was the cause of the mutation. Similarly, a point mutation converting leucine 457 to methionine for the GM638 mutant T allele was found. Moreover, the SV80 line maintained its permissivity for SV40 DNA replication but did not complement the SV40 tsA209 mutant at its nonpermissive temperature. The cloned SV80 T-antigen allele, though replication incompetent, maintained its ability to transform rodent cells at wild-type efficiencies. A compilation of spontaneously occurring SV40 mutations which cannot replicate but can transform shows that these mutations tend to cluster in two regions of the T-antigen gene, one ascribed to the site-specific DNA-binding ability of the protein, and the other to the ATPase activity which is linked to its helicase activity.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botchan M., Stringer J., Mitchison T., Sambrook J. Integration and excision of SV40 DNA from the chromosome of a transformed cell. Cell. 1980 May;20(1):143–152. doi: 10.1016/0092-8674(80)90242-1. [DOI] [PubMed] [Google Scholar]
- Botchan M., Topp W., Sambrook J. Studies on simian virus 40 excision from cellular chromosomes. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):709–719. doi: 10.1101/sqb.1979.043.01.079. [DOI] [PubMed] [Google Scholar]
- Botchan M., Topp W., Sambrook J. The arrangement of simian virus 40 sequences in the DNA of transformed cells. Cell. 1976 Oct;9(2):269–287. doi: 10.1016/0092-8674(76)90118-5. [DOI] [PubMed] [Google Scholar]
- Carroll R. B., Hager L., Dulbecco R. Simian virus 40 T antigen binds to DNA. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3754–3757. doi: 10.1073/pnas.71.9.3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad S. E., Liu C. P., Botchan M. R. Fragment spanning the SV40 replication origin is the only DNA sequence required in cis for viral excision. Science. 1982 Dec 17;218(4578):1223–1225. doi: 10.1126/science.6293055. [DOI] [PubMed] [Google Scholar]
- Cosman D. J., Tevethia M. J. Characterization of a temperature-sensitive, DNA-positive, nontransforming mutant of simian virus 40. Virology. 1981 Jul 30;112(2):605–624. doi: 10.1016/0042-6822(81)90306-8. [DOI] [PubMed] [Google Scholar]
- Fiers W., Contreras R., Haegemann G., Rogiers R., Van de Voorde A., Van Heuverswyn H., Van Herreweghe J., Volckaert G., Ysebaert M. Complete nucleotide sequence of SV40 DNA. Nature. 1978 May 11;273(5658):113–120. doi: 10.1038/273113a0. [DOI] [PubMed] [Google Scholar]
- Flint S. J., Beltz G. A. Expression of transforming viral genes in semipermissive cells transformed by SV40 or adenovirus type 2 to type 5. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):89–102. doi: 10.1101/sqb.1980.044.01.011. [DOI] [PubMed] [Google Scholar]
- GIRARDI A. J., JENSEN F. C., KOPROWSKI H. SV40-INDUCED TRANFORMATION OF HUMAN DIPLOID CELLS: CRISIS AND RECOVERY. J Cell Physiol. 1965 Feb;65:69–83. doi: 10.1002/jcp.1030650110. [DOI] [PubMed] [Google Scholar]
- Gerard R. D., Gluzman Y. New host cell system for regulated simian virus 40 DNA replication. Mol Cell Biol. 1985 Nov;5(11):3231–3240. doi: 10.1128/mcb.5.11.3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluzman Y., Ahrens B. SV40 early mutants that are defective for viral DNA synthesis but competent for transformation of cultured rat and simian cells. Virology. 1982 Nov;123(1):78–92. doi: 10.1016/0042-6822(82)90296-3. [DOI] [PubMed] [Google Scholar]
- Gluzman Y., Frisque R. J., Sambrook J. Origin-defective mutants of SV40. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):293–300. doi: 10.1101/sqb.1980.044.01.033. [DOI] [PubMed] [Google Scholar]
- Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981 Jan;23(1):175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Griffin J. D., Spangler G., Livingston D. M. Enzymatic activities associated with the SV40 large T antigen. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):113–122. doi: 10.1101/sqb.1980.044.01.013. [DOI] [PubMed] [Google Scholar]
- Gruss C., Baumann E., Knippers R. DNA binding properties of a mutant T antigen from the simian virus 40-transformed human cell line simian virus 80. J Virol. 1984 Jun;50(3):943–946. doi: 10.1128/jvi.50.3.943-946.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D., Meselson M. Plasmid screening at high colony density. Gene. 1980 Jun;10(1):63–67. doi: 10.1016/0378-1119(80)90144-4. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Henderson I. C., Livingston D. M. Partial purification and characterization of the SV40 T antigen. Cell. 1974 Sep;3(1):65–70. doi: 10.1016/0092-8674(74)90041-5. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Kimura G., Dulbecco R. A temperature-sensitive mutant of simian virus 40 affecting transforming ability. Virology. 1973 Apr;52(2):529–534. doi: 10.1016/0042-6822(73)90348-6. [DOI] [PubMed] [Google Scholar]
- Kimura G., Dulbecco R. Isolation and characterization of temperature-sensitive mutants of simian virus 40. Virology. 1972 Aug;49(2):394–403. doi: 10.1016/0042-6822(72)90492-8. [DOI] [PubMed] [Google Scholar]
- Kriegler M. P., Griffin J. D., Livingston D. M. Phenotypic complementation of the SV40 tsA mutant defect in viral DNA synthesis following microinjection of SV40 T antigen. Cell. 1978 Aug;14(4):983–994. doi: 10.1016/0092-8674(78)90352-5. [DOI] [PubMed] [Google Scholar]
- Kucherlapati R., Hwang S. P., Shimizu N., McDougall J. K., Botchan M. R. Another chromosomal assignment for a simian virus 40 integration site in human cells. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4460–4464. doi: 10.1073/pnas.75.9.4460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C. J., Nathans D. Mapping temperature-sensitive mutants of simian virus 40: rescue of mutants by fragments of viral DNA. Virology. 1974 Aug;60(2):466–475. doi: 10.1016/0042-6822(74)90340-7. [DOI] [PubMed] [Google Scholar]
- Lai C. J., Nathans D. Mapping the genes of simian virus 40. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):53–60. doi: 10.1101/sqb.1974.039.01.010. [DOI] [PubMed] [Google Scholar]
- Lusky M., Botchan M. Inhibition of SV40 replication in simian cells by specific pBR322 DNA sequences. Nature. 1981 Sep 3;293(5827):79–81. doi: 10.1038/293079a0. [DOI] [PubMed] [Google Scholar]
- Manos M. M., Gluzman Y. Simian virus 40 large T-antigen point mutants that are defective in viral DNA replication but competent in oncogenic transformation. Mol Cell Biol. 1984 Jun;4(6):1125–1133. doi: 10.1128/mcb.4.6.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McCutchan J. H., Pagano J. S. Enchancement of the infectivity of simian virus 40 deoxyribonucleic acid with diethylaminoethyl-dextran. J Natl Cancer Inst. 1968 Aug;41(2):351–357. [PubMed] [Google Scholar]
- Miller J., Bullock P., Botchan M. Simian virus 40 T antigen is required for viral excision from chromosomes. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7534–7538. doi: 10.1073/pnas.81.23.7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami Y., Wobbe C. R., Weissbach L., Dean F. B., Hurwitz J. Role of DNA polymerase alpha and DNA primase in simian virus 40 DNA replication in vitro. Proc Natl Acad Sci U S A. 1986 May;83(9):2869–2873. doi: 10.1073/pnas.83.9.2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers R. M., Rio D. C., Robbins A. K., Tjian R. SV40 gene expression is modulated by the cooperative binding of T antigen to DNA. Cell. 1981 Aug;25(2):373–384. doi: 10.1016/0092-8674(81)90056-8. [DOI] [PubMed] [Google Scholar]
- Myers R. M., Williams R. C., Tjian R. Oligomeric structure of a simian virus 40 T antigen in free form and bound to DNA. J Mol Biol. 1981 Jun 5;148(4):347–353. doi: 10.1016/0022-2836(81)90180-7. [DOI] [PubMed] [Google Scholar]
- Paucha E., Kalderon D., Harvey R. W., Smith A. E. Simian virus 40 origin DNA-binding domain on large T antigen. J Virol. 1986 Jan;57(1):50–64. doi: 10.1128/jvi.57.1.50-64.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pintel D., Bouck N., di Mayorca G., Thimmappaya B., Swerdlow B., Shenk T. SV40 mutant tsA1499 is heat-sensitive for lytic growth but generates cold-sensitive rat-cell transformants. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):305–309. doi: 10.1101/sqb.1980.044.01.035. [DOI] [PubMed] [Google Scholar]
- Risser R., Pollack R. A nonselective analysis of SV40 transformation of mouse 3T3 cells. Virology. 1974 Jun;59(2):477–489. doi: 10.1016/0042-6822(74)90457-7. [DOI] [PubMed] [Google Scholar]
- Smale S. T., Tjian R. T-antigen-DNA polymerase alpha complex implicated in simian virus 40 DNA replication. Mol Cell Biol. 1986 Nov;6(11):4077–4087. doi: 10.1128/mcb.6.11.4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spangler G. J., Griffin J. D., Rubin H., Livingston D. M. Identification and initial characterization of a new low-molecular-weight virus-encoded T antigen in a line of simian virus 40-transformed cells. J Virol. 1980 Nov;36(2):488–498. doi: 10.1128/jvi.36.2.488-498.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringer J. R. Mutant of simian virus 40 large T-antigen that is defective for viral DNA synthesis, but competent for transformation of cultured rat cells. J Virol. 1982 Jun;42(3):854–864. doi: 10.1128/jvi.42.3.854-864.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornow J., Polvino-Bodnar M., Santangelo G., Cole C. N. Two separable functional domains of simian virus 40 large T antigen: carboxyl-terminal region of simian virus 40 large T antigen is required for efficient capsid protein synthesis. J Virol. 1985 Feb;53(2):415–424. doi: 10.1128/jvi.53.2.415-424.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss B., Jacquemin-Sablon A., Live T. R., Fareed G. C., Richardson C. C. Enzymatic breakage and joining of deoxyribonucleic acid. VI. Further purification and properties of polynucleotide ligase from Escherichia coli infected with bacteriophage T4. J Biol Chem. 1968 Sep 10;243(17):4543–4555. [PubMed] [Google Scholar]
- Zouzias D., Jha K. K., Mulder C., Basilico C., Ozer H. L. Human fibroblasts transformed by the early region of SV40 DNA: analysis of "free" viral DNA sequences. Virology. 1980 Jul 30;104(2):439–453. doi: 10.1016/0042-6822(80)90346-3. [DOI] [PubMed] [Google Scholar]