Abstract
Monoclonal antibodies produced against the prototype cell-adapted Wyoming strain of equine infectious anemia virus (EIAV), a lentivirus, were studied for reactivity with the homologous prototype and 16 heterologous isolates. Eighteen hybridomas producing monoclonal antibodies (MAbs) were isolated. Western blot (immunoblot) analyses indicated that 10 were specific for the major envelope glycoprotein (gp90) and 8 for the transmembrane glycoprotein (gp45). Four MAbs specific to epitopes of gp90 neutralized prototype EIAV infectivity. These neutralizing MAbs apparently reacted with variable regions of the envelope gp90, as evidenced by their unique reactivity with the panel of isolates, suggesting recognition of at least three different neutralization epitopes. The conformation of these epitopes appears to be continuous, as they resisted treatment with sodium dodecyl sulfate and reducing reagents. Monoclonal antibodies that reacted with conserved epitopes on gp90 or gp45 failed to neutralize EIAV. Our data also demonstrated that there was a large spectrum of possible EIAV serotypes and confirmed that antigenic variation occurs with high frequency in EIAV. Moreover, the data showed that variation is a rapid and random process, as no pattern of variant evolution was evident by comparison of 13 isolates from parallel infections. These results represent the first production of neutralizing MAbs specific for a lentivirus glycoprotein and document alterations in one or more neutralization epitopes of the major surface glycoprotein among sequential isolates of EIAV recovered during persistent infection.
Full text
PDF
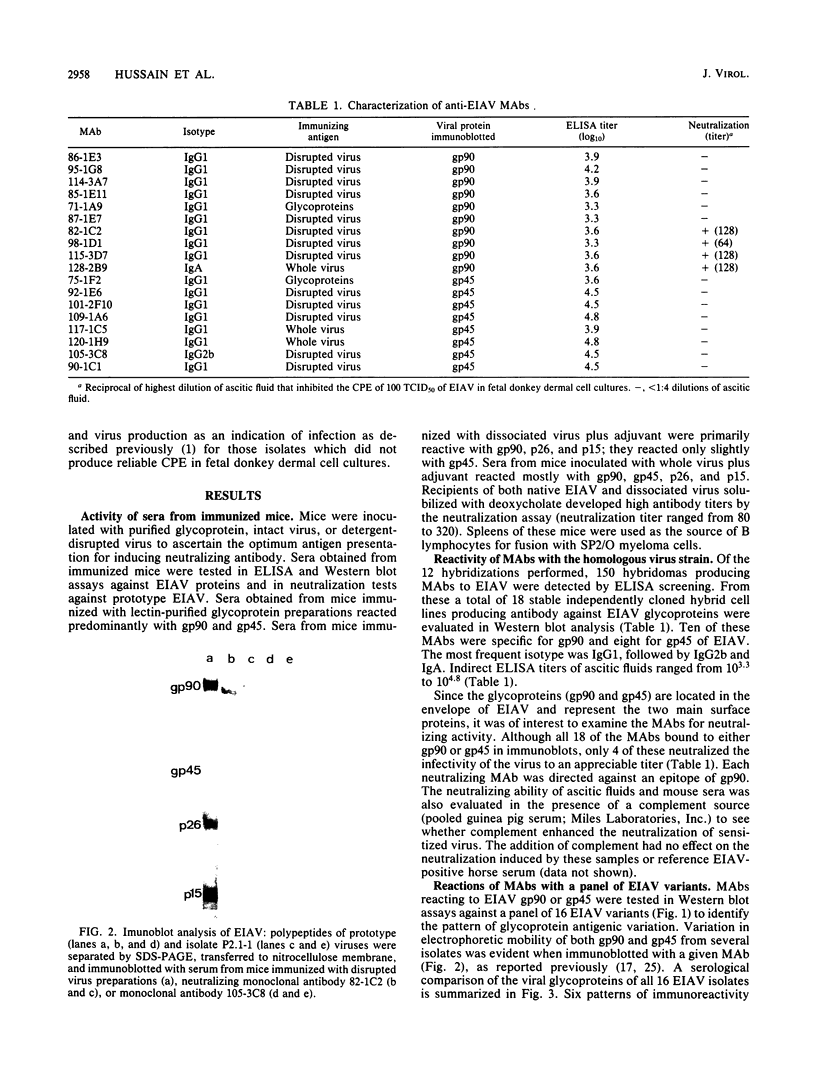
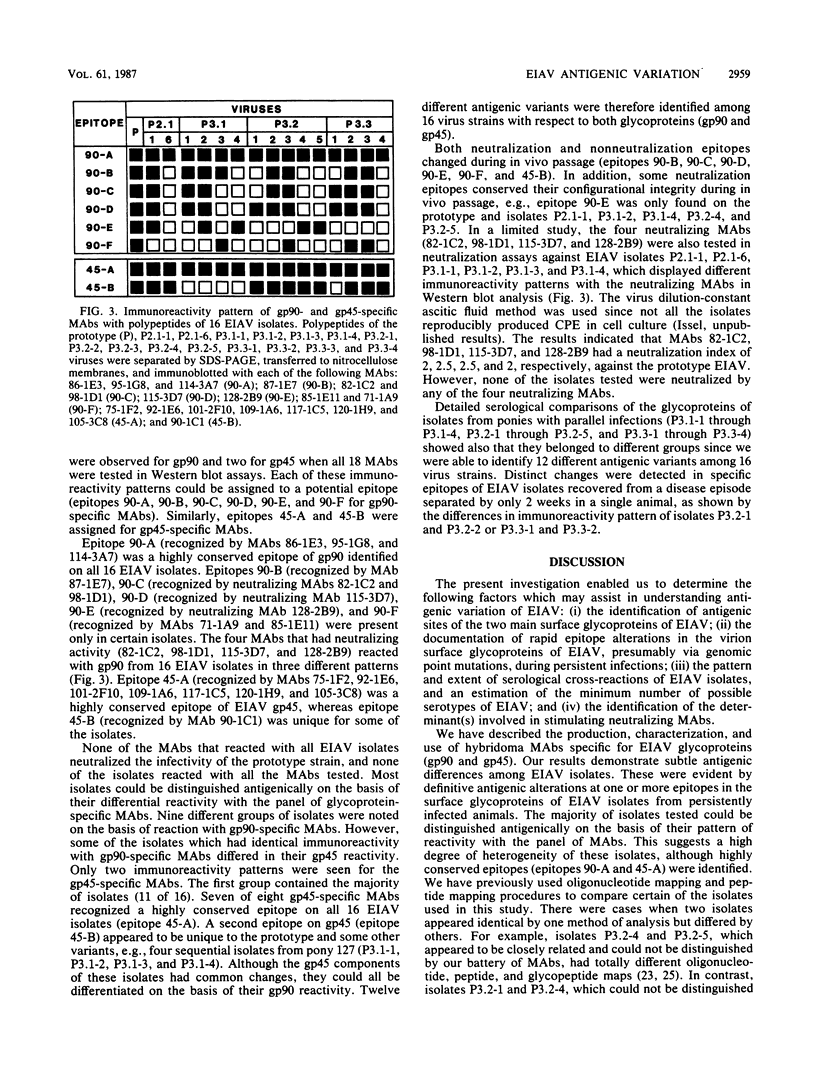


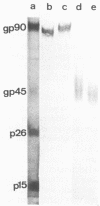
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amborski G. F., Jeffers G., Amborski R. L., Issel C. J. Equine infectious anemia virus: development of a simple reproducible method for titrating infectivity of the cell-adapted strain. Am J Vet Res. 1979 Feb;40(2):302–304. [PubMed] [Google Scholar]
- Benn S., Rutledge R., Folks T., Gold J., Baker L., McCormick J., Feorino P., Piot P., Quinn T., Martin M. Genomic heterogeneity of AIDS retroviral isolates from North America and Zaire. Science. 1985 Nov 22;230(4728):949–951. doi: 10.1126/science.2997922. [DOI] [PubMed] [Google Scholar]
- Blondel B., Crainic R., Fichot O., Dufraisse G., Candrea A., Diamond D., Girard M., Horaud F. Mutations conferring resistance to neutralization with monoclonal antibodies in type 1 poliovirus can be located outside or inside the antibody-binding site. J Virol. 1986 Jan;57(1):81–90. doi: 10.1128/jvi.57.1.81-90.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Clements J. E., D'Antonio N., Narayan O. Genomic changes associated with antigenic variation of visna virus. II. Common nucleotide sequence changes detected in variants from independent isolations. J Mol Biol. 1982 Jul 5;158(3):415–434. doi: 10.1016/0022-2836(82)90207-8. [DOI] [PubMed] [Google Scholar]
- Clements J. E., Pedersen F. S., Narayan O., Haseltine W. A. Genomic changes associated with antigenic variation of visna virus durig persistent infection. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4454–4458. doi: 10.1073/pnas.77.8.4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimmock N. J. Mechanisms of neutralization of animal viruses. J Gen Virol. 1984 Jun;65(Pt 6):1015–1022. doi: 10.1099/0022-1317-65-6-1015. [DOI] [PubMed] [Google Scholar]
- Galfrè G., Milstein C. Preparation of monoclonal antibodies: strategies and procedures. Methods Enzymol. 1981;73(Pt B):3–46. doi: 10.1016/0076-6879(81)73054-4. [DOI] [PubMed] [Google Scholar]
- Issel C. J., Coggins L. Equine infectious anemia: current knowledge. J Am Vet Med Assoc. 1979 Apr 1;174(7):727–733. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Malmquist W. A., Barnett D., Becvar C. S. Production of equine infectious anemia antigen in a persistently infected cell line. Arch Gesamte Virusforsch. 1973;42(4):361–370. doi: 10.1007/BF01250717. [DOI] [PubMed] [Google Scholar]
- Montelaro R. C., Bolognesi D. P. Structure and morphogenesis of type-C retroviruses. Adv Cancer Res. 1978;28:63–89. doi: 10.1016/s0065-230x(08)60646-6. [DOI] [PubMed] [Google Scholar]
- Montelaro R. C., Lohrey N., Parekh B., Blakeney E. W., Issel C. J. Isolation and comparative biochemical properties of the major internal polypeptides of equine infectious anemia virus. J Virol. 1982 Jun;42(3):1029–1038. doi: 10.1128/jvi.42.3.1029-1038.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montelaro R. C., Parekh B., Orrego A., Issel C. J. Antigenic variation during persistent infection by equine infectious anemia virus, a retrovirus. J Biol Chem. 1984 Aug 25;259(16):10539–10544. [PubMed] [Google Scholar]
- Montelaro R. C., West M., Issel C. J. Isolation of equine infectious anemia virus glycoproteins. Lectin affinity chromatography procedures for high avidity glycoproteins. J Virol Methods. 1983 Jun;6(6):337–346. doi: 10.1016/0166-0934(83)90056-3. [DOI] [PubMed] [Google Scholar]
- Narayan O., Griffin D. E., Clements J. E. Virus mutation during 'slow infection': temporal development and characterization of mutants of visna virus recovered from sheep. J Gen Virol. 1978 Nov;41(2):343–352. doi: 10.1099/0022-1317-41-2-343. [DOI] [PubMed] [Google Scholar]
- O'Sullivan M. J., Gnemmi E., Morris D., Chieregatti G., Simmonds A. D., Simmons M., Bridges J. W., Marks V. Comparison of two methods of preparing enzyme-antibody conjugates: application of these conjugates for enzyme immunoassay. Anal Biochem. 1979 Nov 15;100(1):100–108. doi: 10.1016/0003-2697(79)90117-9. [DOI] [PubMed] [Google Scholar]
- Orrego A., Issel C. J., Montelaro R. C., Adams W. V., Jr Virulence and in vitro growth of a cell-adapted strain of equine infectious anemia virus after serial passage in ponies. Am J Vet Res. 1982 Sep;43(9):1556–1560. [PubMed] [Google Scholar]
- Parekh B., Issel C. J., Montelaro R. C. Equine infectious anemia virus, a putative lentivirus, contains polypeptides analogous to prototype-C oncornaviruses. Virology. 1980 Dec;107(2):520–525. doi: 10.1016/0042-6822(80)90319-0. [DOI] [PubMed] [Google Scholar]
- Payne S. L., Salinovich O., Nauman S. M., Issel C. J., Montelaro R. C. Course and extent of variation of equine infectious anemia virus during parallel persistent infections. J Virol. 1987 Apr;61(4):1266–1270. doi: 10.1128/jvi.61.4.1266-1270.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne S., Parekh B., Montelaro R. C., Issel C. J. Genomic alterations associated with persistent infections by equine infectious anaemia virus, a retrovirus. J Gen Virol. 1984 Aug;65(Pt 8):1395–1399. doi: 10.1099/0022-1317-65-8-1395. [DOI] [PubMed] [Google Scholar]
- Ratner L., Gallo R. C., Wong-Staal F. HTLV-III, LAV, ARV are variants of same AIDS virus. Nature. 1985 Feb 21;313(6004):636–637. doi: 10.1038/313636c0. [DOI] [PubMed] [Google Scholar]
- Salinovich O., Payne S. L., Montelaro R. C., Hussain K. A., Issel C. J., Schnorr K. L. Rapid emergence of novel antigenic and genetic variants of equine infectious anemia virus during persistent infection. J Virol. 1986 Jan;57(1):71–80. doi: 10.1128/jvi.57.1.71-80.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer W., Bolognesi D. P. Mammalian C-type oncornaviruses: relationships between viral structural and cell-surface antigens and their possible significance in immunological defense mechanisms. Contemp Top Immunobiol. 1977;6:127–167. doi: 10.1007/978-1-4684-3051-6_4. [DOI] [PubMed] [Google Scholar]
- Scott J. V., Stowring L., Haase A. T., Narayan O., Vigne R. Antigenic variation in visna virus. Cell. 1979 Oct;18(2):321–327. doi: 10.1016/0092-8674(79)90051-5. [DOI] [PubMed] [Google Scholar]
- Shulman M., Wilde C. D., Köhler G. A better cell line for making hybridomas secreting specific antibodies. Nature. 1978 Nov 16;276(5685):269–270. doi: 10.1038/276269a0. [DOI] [PubMed] [Google Scholar]
- Towbin H., Gordon J. Immunoblotting and dot immunobinding--current status and outlook. J Immunol Methods. 1984 Sep 4;72(2):313–340. doi: 10.1016/0022-1759(84)90001-2. [DOI] [PubMed] [Google Scholar]
- Wong-Staal F., Shaw G. M., Hahn B. H., Salahuddin S. Z., Popovic M., Markham P., Redfield R., Gallo R. C. Genomic diversity of human T-lymphotropic virus type III (HTLV-III). Science. 1985 Aug 23;229(4715):759–762. doi: 10.1126/science.2992084. [DOI] [PubMed] [Google Scholar]