Abstract
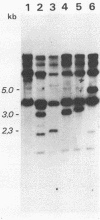
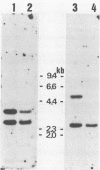
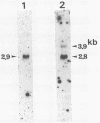
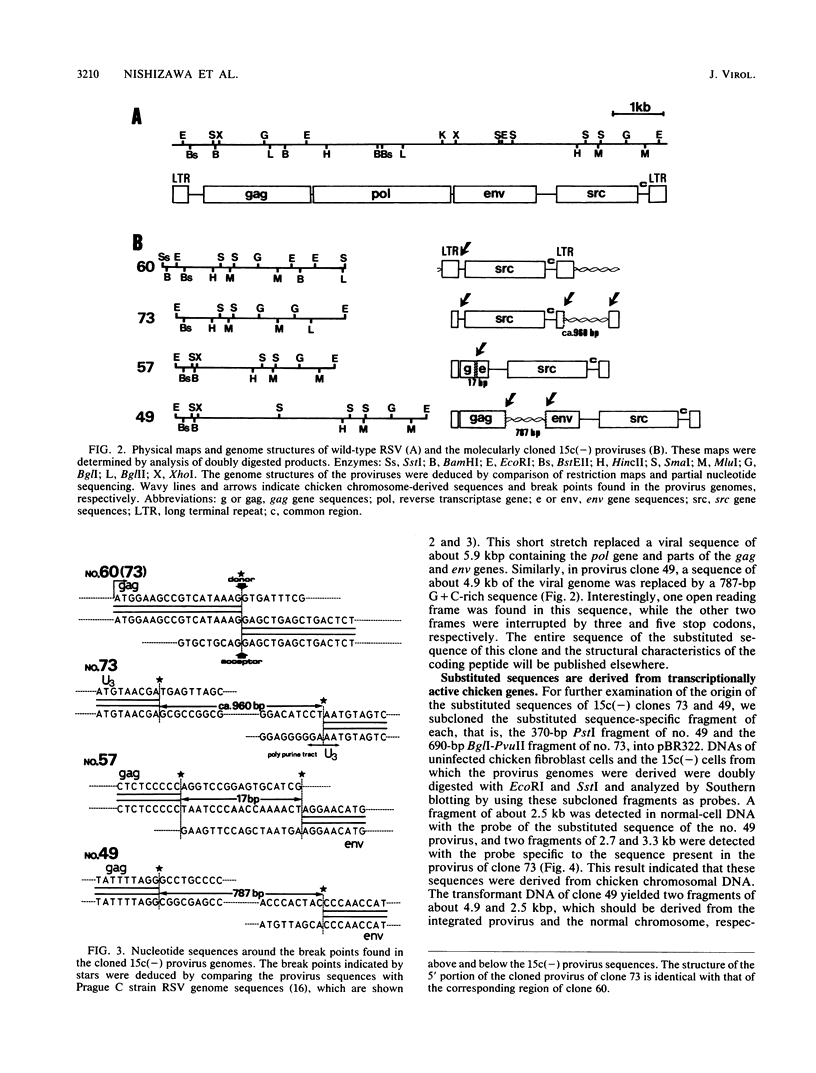
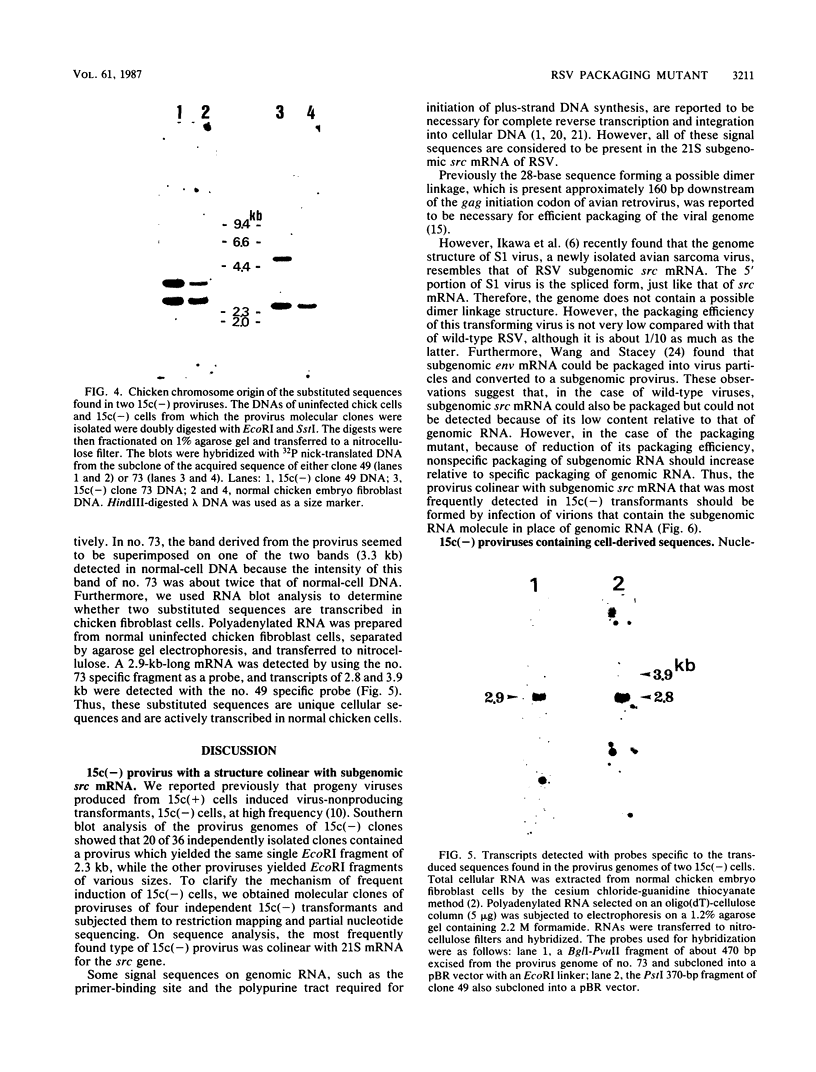
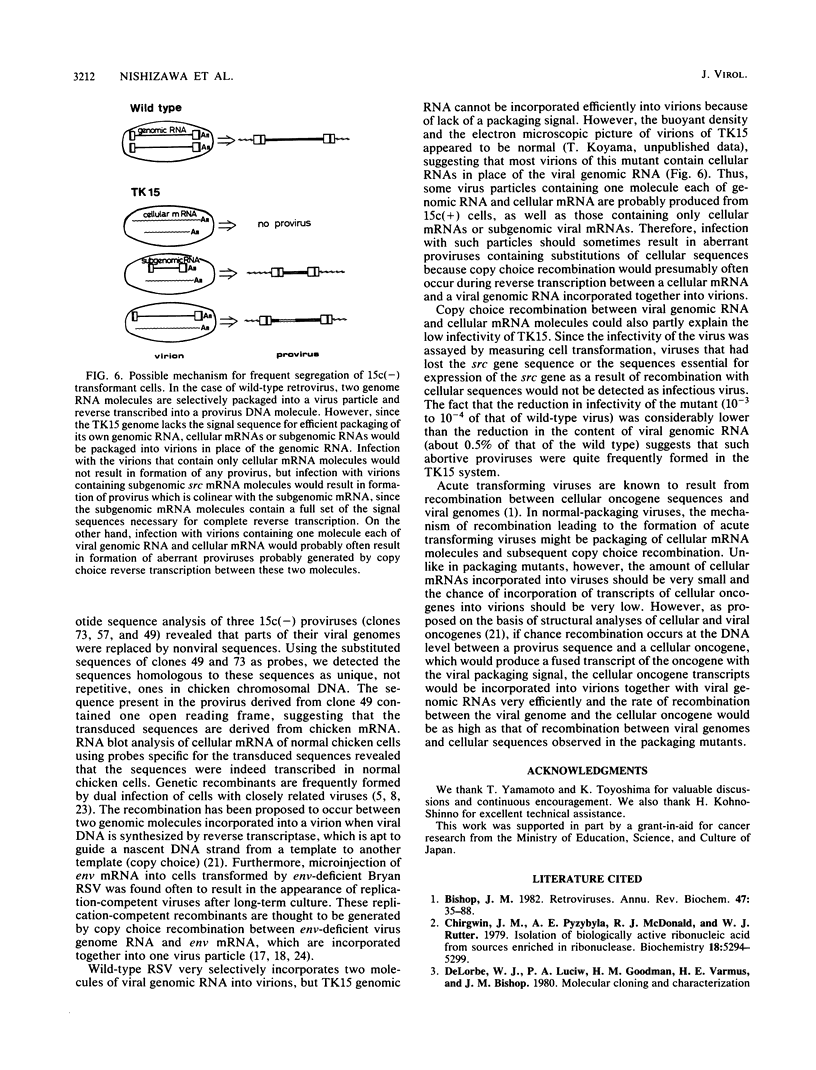
TK15, a mutant derived from a temperature-sensitive mutant of Rous sarcoma virus (tsNY68), has extremely low infectivity although it has intact viral genes. Previous analyses of the virus and virus-induced transformants showed that the mutant has a defect in packaging of its own genomic RNA, possibly owing to a deletion near the 5' end. Another striking feature of TK15 is that it induces various types of virus-nonproducing (NP) transformants, 15c(-), at high frequency. In this work, the mechanisms of frequent segregation of NP cells were examined by molecular cloning of TK15-derived proviruses from NP cell clones and their sequence analysis. The structure of the major type of provirus, found in about half of the NP cell clones, was colinear with src subgenomic mRNA and was suggested to be due to infection with virions containing subgenomic mRNA in place of genomic RNA. Other types of proviruses present in 15c(-) cells appeared to contain cellular sequences of various lengths replacing various parts of viral sequences. The mechanism for the generation of these proviruses is discussed in relation to the nature of the packaging mutant.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop J. M. Retroviruses. Annu Rev Biochem. 1978;47:35–88. doi: 10.1146/annurev.bi.47.070178.000343. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- DeLorbe W. J., Luciw P. A., Goodman H. M., Varmus H. E., Bishop J. M. Molecular cloning and characterization of avian sarcoma virus circular DNA molecules. J Virol. 1980 Oct;36(1):50–61. doi: 10.1128/jvi.36.1.50-61.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enquist L., Sternberg N. In vitro packaging of lambda Dam vectors and their use in cloning DNA fragments. Methods Enzymol. 1979;68:281–298. doi: 10.1016/0076-6879(79)68020-5. [DOI] [PubMed] [Google Scholar]
- Hunter E. The mechanism for genetic recombination in the avian retroviruses. Curr Top Microbiol Immunol. 1978;79:295–309. doi: 10.1007/978-3-642-66853-1_7. [DOI] [PubMed] [Google Scholar]
- Ikawa S., Hagino-Yamagishi K., Kawai S., Yamamoto T., Toyoshima K. Activation of the cellular src gene by transducing retrovirus. Mol Cell Biol. 1986 Jul;6(7):2420–2428. doi: 10.1128/mcb.6.7.2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai S., Hanafusa H. Genetic recombination with avian tumor virus. Virology. 1972 Jul;49(1):37–44. doi: 10.1016/s0042-6822(72)80005-9. [DOI] [PubMed] [Google Scholar]
- Kawai S., Hanafusa H. Recombination between a temperature-sensitive mutant and a deletion mutant of Rous sarcoma virus. J Virol. 1976 Aug;19(2):389–397. doi: 10.1128/jvi.19.2.389-397.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai S., Hanafusa H. The effects of reciprocal changes in temperature on the transformed state of cells infected with a rous sarcoma virus mutant. Virology. 1971 Nov;46(2):470–479. doi: 10.1016/0042-6822(71)90047-x. [DOI] [PubMed] [Google Scholar]
- Kawai S., Koyama T. Characterization of a Rous sarcoma virus mutant defective in packaging its own genomic RNA: biological properties of mutant TK15 and mutant-induced transformants. J Virol. 1984 Jul;51(1):147–153. doi: 10.1128/jvi.51.1.147-153.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama T., Harada F., Kawai S. Characterization of a Rous sarcoma virus mutant defective in packaging its own genomic RNA: biochemical properties of mutant TK15 and mutant-induced transformants. J Virol. 1984 Jul;51(1):154–162. doi: 10.1128/jvi.51.1.154-162.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Nishizawa M., Koyama T., Kawai S. Unusual features of the leader sequence of Rous sarcoma virus packaging mutant TK15. J Virol. 1985 Sep;55(3):881–885. doi: 10.1128/jvi.55.3.881-885.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizawa M., Mayer B. J., Takeya T., Yamamoto T., Toyoshima K., Hanafusa H., Kawai S. Two independent mutations are required for temperature-sensitive cell transformation by a Rous sarcoma virus temperature-sensitive mutant. J Virol. 1985 Dec;56(3):743–749. doi: 10.1128/jvi.56.3.743-749.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugatsch T., Stacey D. W. Identification of a sequence likely to be required for avian retroviral packaging. Virology. 1983 Jul 30;128(2):505–511. doi: 10.1016/0042-6822(83)90279-9. [DOI] [PubMed] [Google Scholar]
- Schwartz D. E., Tizard R., Gilbert W. Nucleotide sequence of Rous sarcoma virus. Cell. 1983 Mar;32(3):853–869. doi: 10.1016/0092-8674(83)90071-5. [DOI] [PubMed] [Google Scholar]
- Stacey D. W., Allfrey V. G., Hanafusa H. Microinjection analysis of envelope-glycoprotein messenger activities of avian leukosis viral RNAs. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1614–1618. doi: 10.1073/pnas.74.4.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacey D. W. Expression of a subgenomic retroviral messenger RNA. Cell. 1980 Oct;21(3):811–820. doi: 10.1016/0092-8674(80)90444-4. [DOI] [PubMed] [Google Scholar]
- Swanstrom R., Varmus H. E., Bishop J. M. Nucleotide sequence of the 5' noncoding region and part of the gag gene of Rous sarcoma virus. J Virol. 1982 Feb;41(2):535–541. doi: 10.1128/jvi.41.2.535-541.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varmus H. E. Form and function of retroviral proviruses. Science. 1982 May 21;216(4548):812–820. doi: 10.1126/science.6177038. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Gillespie D. Preparative and analytical purification of DNA from agarose. Proc Natl Acad Sci U S A. 1979 Feb;76(2):615–619. doi: 10.1073/pnas.76.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt P. K. Genetically stable reassortment of markers during mixed infection with avian tumor viruses. Virology. 1971 Dec;46(3):947–952. doi: 10.1016/0042-6822(71)90093-6. [DOI] [PubMed] [Google Scholar]
- Wang L. H., Stacey D. W. Participation of subgenomic retroviral mRNAs in recombination. J Virol. 1982 Mar;41(3):919–930. doi: 10.1128/jvi.41.3.919-930.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]