Abstract
The heat-shock protein 90 (Hsp90) is a cytosolic molecular chaperone that is highly abundant even at normal temperature. Specific functions for Hsp90 have been proposed based on the characterization of its interactions with certain transcription factors and kinases including Raf in vertebrates and flies. We therefore decided to address the role of Hsp90 for MAP kinase pathways in the budding yeast, an organism amenable to both genetic and biochemical analyses. We found that both basal and induced activities of the pheromone-signaling pathway depend on Hsp90. Signaling is defective in strains expressing low levels or point mutants of yeast Hsp90 (Hsp82), or human Hsp90β instead of the wild-type protein. Ste11, a yeast equivalent of Raf, forms complexes with wild-type Hsp90 and depends on Hsp90 function for accumulation. For budding yeast, Ste11 represents the first identified endogenous “substrate” of Hsp90. Moreover, Hsp90 functions in steroid receptor and pheromone signaling can be genetically separated as the Hsp82 point mutant T525I and the human Hsp90β are specifically defective for the former and the latter, respectively. These findings further corroborate the view that molecular chaperones must also be considered as transient or stable components of signal transduction pathways.
INTRODUCTION
The 90-kDa heat-shock protein (Hsp90)1 (for reviews, see Jakob and Buchner, 1994; Csermely et al., 1998) is an ubiquitous and abundantly expressed cytosolic protein even at normal temperature. It is highly conserved from bacteria to mammals. Two genes encode closely related isoforms in mammals as well as in the budding yeast Saccharomyces cerevisiae. Deletion experiments in yeast have shown that the expression of at least one of the two Hsp90 isoforms, either Hsp82 or Hsc82, is essential for viability (Borkovich et al., 1989). Similarly, many mutant alleles of the Drosophila HSP90 homolog, HSP83, are embryonic lethals over a deficiency of the locus (van der Straten et al., 1997), whereas the Escherichia coli homolog of Hsp90, HtpG, appears to be dispensable (Bardwell and Craig, 1988). Hsp90 can act as a molecular chaperone in vitro to promote refolding of denatured proteins (Wiech et al., 1992; Yonehara et al., 1996; see also Shaknovich et al., 1992; Shue and Kohtz, 1994), to hold denatured proteins in a folding-competent state for other chaperones (Freeman and Morimoto, 1996; Yonehara et al., 1996) and to prevent protein unfolding and aggregation (Miyata and Yahara, 1992; Jakob et al., 1995a, 1995b; Yonehara et al., 1996).
The interaction of Hsp90 with steroid receptors, which can be thought of as a signal transduction complex, has been the most extensively investigated. A variety of in vitro and in vivo studies have revealed that steroid receptors are complexed with Hsp90 and several other proteins in the absence of hormone (for review, see Pratt and Toft, 1997). Upon ligand binding, the hormone binding domain (HBD) undergoes a conformational change that results in the release of Hsp90 and the concomitant activation of the steroid receptor. Steroid receptors and many heterologous proteins fused to the HBD are maintained inactive in the absence of hormone. We have therefore hypothesized that the hormone-reversible inactivation function of the HBD is mediated by Hsp90, possibly by steric hindrance (Picard, 1993, 1994). Further insights into the role of Hsp90 in the regulation of this particular signal transduction pathway come from studies made in yeast (reviewed in Picard, 1998). Vertebrate steroid receptors expressed in yeast strains with a low level (Picard et al., 1990; see also Holley and Yamamoto, 1995) or specific point mutants of Hsp82 (Bohen and Yamamoto, 1993; Bohen, 1995; Nathan and Lindquist, 1995; Fang et al., 1996) show a defective hormonal response that is due to a decrease in the ligand-binding affinity (Bohen, 1995; Fang et al., 1996). Thus, Hsp90 may have a dual role: it ensures that receptors are kept inactive in the absence of hormone and helps them to respond specifically and efficiently to ligand. This view is also corroborated by pharmacological in vivo experiments with geldanamycin (Whitesell et al., 1994), a compound that interferes with certain Hsp90 functions such as the proper maturation of steroid receptor–Hsp90 complexes (Smith et al., 1995; Whitesell and Cook, 1996; Bamberger et al., 1997; Czar et al., 1997; Segnitz and Gehring, 1997).
There is ample evidence for a role of Hsp90 in regulating the activity of several other signaling pathways, such as the xenobiotic response mediated by the dioxin receptor (see for example Pongratz et al., 1992; Carver et al., 1994; McGuire et al., 1994; Antonsson et al., 1995; Coumailleau et al., 1995; Whitelaw et al., 1995). Interaction of the dioxin receptor with Hsp90 is essential for ligand binding and for acquiring a DNA-binding conformation. Activation of the dioxin receptor depends on the release of Hsp90 upon ligand binding and heterodimerization with Arnt. A functional dependence on, and a direct interaction with, Hsp90 has also been described for kinases such as the fission yeast Wee1 (Aligue et al., 1994), the vertebrate v-Src (Schuh et al., 1985; Xu and Lindquist, 1993; Nathan and Lindquist, 1995), and the related kinase Lck (Hartson et al., 1996).
Hsp90 may also be required for growth factor signaling. 1) Raf-1, a serine/threonine kinase involved in mitogenic signal transduction in vertebrates, exists in a geldanamycin-sensitive heterocomplex with Hsp90 (Stancato et al., 1993, 1994; Lovric et al., 1994; Wartmann and Davis, 1994; Schulte et al., 1995, 1996; Stancato et al., 1997). 2) Mutations in Drosophila HSP83 reduce signaling by the torso (Doyle and Bishop, 1993) and sevenless receptors (Cutforth and Rubin, 1994; van der Straten et al., 1997), which may be due, at least in part, to a requirement for Hsp90 for Raf function (van der Straten et al., 1997). 3) The insulin receptor binds Hsp90, and antibodies to Hsp90 interfere with insulin signaling (Takata et al., 1997).
Comparable MAPK pathways also exist in yeast where they regulate the pheromone response, invasive growth, pseudohyphal development, osmoregulation, cell wall integrity, and sporulation (for reviews, see Herskowitz, 1995; Levin and Errede, 1995; Schultz et al., 1995; Leberer et al., 1997). The pheromone-signaling pathway has received a lot of attention over the past few years. Binding of the mating pheromones to transmembrane receptors elicits a series of events including the sequential activation of the kinases Ste11, Ste7, and Fus3, leading to morphological changes, a cell cycle arrest in G1, and the expression of specific genes required for mating. The kinase Ste11 from S. cerevisiae occupies a position analogous to that of Raf. This prompted us to test genetically whether Hsp90 plays a role in the pheromone pathway.
MATERIALS AND METHODS
Plasmids
Hsp90 plasmids.
Wild-type Hsp82 (Hsp82 wt), Hsp82 G313N, and Hsp82 T525I were expressed from plasmids pTCA/Hsp82, pTCA/Hsp82 G313N, and pTCA/Hsp82 T525I, respectively (Bohen, 1995), or various derivatives thereof with other auxotrophic markers. Unless indicated, the strong constitutive promoter from the glyceraldehyde-3-phosphate dehydrogenase (GPD) gene TDH3 was used to drive expression. Plasmid pHCA/Hsp82 is the HIS3 version of pTCA/Hsp82 obtained by substituting the backbone of shuttle vector pRS313 for that of pRS314 (Sikorski and Hieter, 1989). Plasmid p2U/Hsp82, a 2μ-URA3 expression vector for Hsp82 has been described previously (Louvion et al., 1996).
To obtain reduced levels of Hsp82 (∼10% of Hsp82 + Hsc82 in a wild-type strain), Hsp82 was expressed from a construct containing the leaky GAL1 promoter from strain GRS4 (Picard et al., 1990) fused to HSP82 coding sequences in plasmid pRS304 (Sikorski and Hieter, 1989). On medium with 2% glucose, repression of this mutant GAL1 promoter construct is only partial, and low levels of Hsp82 accumulate.
Plasmid p2TG/hHsp90β expressing human Hsp90β was constructed as follows. The coding sequence for human Hsp90β was excised as a SnaBI–SalI fragment from pKN1–3 (Rebbe et al., 1987) and cloned into the SmaI site of pSP64 to add a BamHI site at the 5′-end and a SacI site at the 3′-end. The BamHI–SacI fragment containing the human HSP90β sequence was fused to the GPD promoter in shuttle vector pRS304 (Sikorski and Hieter, 1989) with a 2μ replicon. Plasmid p2HG/hHsp90β is the HIS3 version based on expression vector p2HG (Picard et al., 1990).
Plasmids p2G/Hsp82, p2G/Hsp82 G313N, and p2G/hHsp90β are identical to plasmids p2HG/Hsp82 (Louvion et al., 1996), p2HG/Hsp82 G313N (the G313N derivative of p2HG/Hsp82), and p2HG/hHsp90β, respectively, except that they lack an internal HindIII fragment of the HIS3 marker. Thus, rather than an auxotrophic marker it is the Hsp90 function itself that provides the selectable marker for these plasmids.
Plasmid p2TG/flag.Hsp82wt serves to express Hsp82 with a FLAG epitope at the N terminus. The expression vector was derived from p2TG/hHsp90β. Sequences encoding the FLAG epitope (DYKDDDDK) were placed between the initiator codon and the second codon of the wild-type HSP82 sequences, following the introduction of a BglII site just upstream of the second nucleotide of the HSP82-coding sequence. FLAG epitope and second amino acid of Hsp82 are thus separated by the three extra amino acids EIL.
Other Plasmids.
Plasmid pYES/Ste11ΔN encoding Ste11ΔN was generated as follows: the coding sequence for the catalytic domain of Ste11 was excised from plasmid pNC199 (a gift from B. Errede) as a DdeI–BglII fragment and subcloned into pSP72 to add a BamHI site at the 5′-end. This fragment was further subcloned as a BamHI–BglII fragment into a pUC18 derivative containing a stop codon in the proper reading frame followed by a SacI site. Finally, the sequence encoding the catalytic domain of Ste11 was introduced into plasmid pYES 2.0 (Invitrogen, San Diego, CA) as a BamHI–SacI fragment. pYES 2.0 is a yeast expression vector that contains the galactose-inducible GAL1 promoter, the 2μ replicon, and the URA3 selectable marker. Plasmid pYES/HA-Ste11 was constructed for galactose-inducible expression of full-length Ste11 with an influenza virus hemagglutinin (HA) epitope (Daro et al., 1996) at its N terminus; a KpnI–NdeI fragment with sequences encoding the HA epitope (MQDLPGNDNSTAG) was joined in-frame to a BamHI fragment carrying STE11-coding sequences from plasmid BB345 (mentioned as pYBS345 in Choi et al., 1994) and cloned into pYES 2.0 linearized with KpnI and NotI; noncomplementary sites were filled in or chewed back to allow ligation.
Plasmid pUCA/Ste7 M was used to express a myc-tagged Ste7 protein. It contains the CYC1 promoter and Ste7-coding sequences from plasmid pNC318 (Zhou et al., 1993) excised as a SalI–HindIII fragment and cloned into the SalI–SmaI linearized plasmid pRS316 (Sikorski and Hieter, 1989).
The yeast genomic library (a gift obtained via M. Collart) was a Sau 3A partial library cloned into the BamHI site of the 2μ-URA3 vector YEplac195 (Gietz and Sugino, 1988).
Plasmids p2U/GST-2 (Warth et al., 1997), p2U/GST-STE5, and pYes/Ste11ΔN.GST served to express glutathione-S-transferase (GST), GST fused to Ste5, and GST fused to Ste11ΔN, respectively. p2U/GST-STE5 was constructed by replacing the BamHI–BglII fragment at the 5′-end of HSP82 of p2U/Hsp82 with a BamHI fragment carrying GST-coding sequences fused in-frame to STE5 sequences; STE5 sequences lacking the first 24 codons were from plasmid BB192 (mentioned as pYBS146 in Choi et al., 1994).
Strains
The parent strains and some of the derivatives are listed in Table 2. The related yeast strain backgrounds, HH1a and JC6a (gifts from S. Lindquist), were used to replace the endogenous Hsp82/Hsc82 with Hsp90 mutants by plasmid shuffling. Plasmids were introduced into yeast by the LiAc/PEG method and selected for on appropriate minimal media. Strain HH1a-pHCA/Hsp82wt is essentially the MATa version of the previously described strain HH1-KAT6 (see Palmer et al., 1995). It was obtained by tetrad dissection of a diploidized HH1-KAT6 and further plasmid shuffling.
Table 2.
Yeast strains
| Strain | Genotypea | Source |
|---|---|---|
| HH1-KAT6 | MATα ade2-1 can1-100 his3-12,16 leu2-3,112 trp1-1 ura3-1 Δhsc82::LEU2 Δhsp82::LEU2 / GAL1-hHSP90β-CEN/ARS-HIS3 [pGAL1-hhsp90] | S. Lindquist (described in Palmer et al., 1995) |
| HH1ab | MATa ade2-1 can1-100 his3-12,16 leu2-3,112 trp1-1 ura3-1 Δhsc82::LEU2 Δhsp82::LEU2 | This article |
| HH1a-pHCA/Hsp82wt | HH1a / HSP82-CEN/ARS-HIS3 [pHCA/Hsp82] | This article |
| DP120 | HH1a / HSP82-2μ-URA3 [p2U/Hsp82] | This article |
| DP121 | DP120 / fus1::HIS3 | This article |
| DP122 | HH1a / fus1::HIS3 / HSP82-CEN/ARS-TRP1 [pTCA/Hsp82] | This article |
| DP123 | HH1a / fus1::HIS3 / HSP82 G313N-CEN/ARS-TRP1 [pTCA/Hsp82 G313N] | This article |
| DP124 | HH1a / fus1::HIS3 / hHSP90β-2μ-TRP1 [p2TG/hHsp90β] | This article |
| HH1a-p2G/Hsp82wt | HH1a / HSP82-2μ [p2G/Hsp82] | This article |
| HH1a-p2G/Hsp82 G313N | HH1a / HSP82 G313N-2μ [p2G/Hsp82 G313N] | This article |
| HH1a-p2G/hHsp90β | HH1a / hHSP90β-2μ [p2G/hHsp90β] | This article |
| HH1a-p2TG/flag.Hsp82wt | HH1a / flag.Hsp82-2μ-TRP1 [p2TG/flag.Hsp82wt] | This article |
| JC6ab | MATa ade2-1 can1-100 his3-11,15 leu2-3,112 trp1-1 ura3-1 Δhsc82::LEU2 Δhsp82::LEU2 Δpep4::HIS3 | S. Lindquist |
| JC6a-Hsp82 | JC6a / HSP82-CEN/ARS-TRP1 [pTCA/Hsp82] | This article |
| JC6a-Hsp82 G313N | JC6a / HSP82 G313N-CEN/ARS-TRP1 [pTCA/Hsp82 G313N] | This article |
| JC6a-hHsp90β | JC6a / hHSP90β-2μ-TRP1 [p2TG/hHsp90β] | This article |
| RMY326 | MATa his3 leu2-3,112 trp1-1 ura3-52 | R. Movva |
| E929-6C-20 | E929-6C-0 ste11-Δ6 | Rhodes et al., 1990 |
Episomes are indicated after a slash with the name of the plasmid in brackets.
HH1a and JC6a represent related genotypic backgrounds of many strains rather than real strains (they are not viable without Hsp90 function being provided, for example, by a plasmid).
The strain DP121 was obtained by substituting the HIS3 coding body for that of FUS1 in strain DP120 (see Table 2) with the gene replacement construct pSL1497 (Stevenson et al., 1992). Plasmid p2U/Hsp82 was subsequently replaced by the Hsp90 expression vectors pTCA/Hsp82, pTCA/Hsp82 G313N, and p2TG/hHsp90β, to yield strains DP122, DP123, and DP124, respectively. In strains HH1a-p2G/Hsp82wt, HH1a-p2G/Hsp82 G313N, and HH1a-p2G/hHsp90, the Hsp90 derivatives themselves are used as selectable marker to maintain the episomes.
α-Factor Induction
To monitor the cell cycle arrest in response to α-factor, cells were diluted to a density of 1.2 × 107 cells/ml and streaked or spotted onto YEPD plates containing 10 mM Na-citrate, pH 4.3, and, where indicated, 5 μM α-factor (Bachem, Torrance, CA). The FUS1-LacZ reporter plasmid pSB234 was used to measure the transcriptional output of the pheromone pathway (Trueheart et al., 1987). Wild-type and mutant strains were grown to early logarithmic phase and exposed to 5 μM α-factor for 2 h after addition of 10 mM Na-citrate, pH 4.3. Quantification of the LacZ expression was performed as described by Yocum et al. (1984) except that chlorophenol red-β-d-galactopyranoside was used as β-galactosidase substrate instead of O-nitrophenyl β-d-galactopyranoside for more sensitivity.
Rapid Protein Extraction
The levels of overexpressed Ste11 (yeast strains JC6a-Hsp82, JC6a-Hsp82 G313N, and JC6a-hHsp90β with plasmid pYES/HA-Ste11) were quantitated using crude extracts prepared by a rapid protein extraction protocol (Horvath and Riezman, 1994) and loaded onto 10% SDS-polyacrylamide gels. To confirm that equal amounts of protein had been loaded, proteins were stained with Ponceau S after transfer onto a nitrocellulose membrane before immunostaining.
Analysis of Ste7 Phosphorylation
JC6a strains expressing the Hsp90 derivatives were transformed with plasmid pUCA/Ste7 M. Transformants were grown to early logarithmic phase in 1% sucrose as a carbon source. After addition of 10 mM Na-citrate, pH 4.3, the cultures were exposed to 5 μM α-factor for 2 h. Cell extracts were prepared at 4°C by breaking the cells with glass beads in 10 mM Tris-HCl, pH 7.5, 50 mM NaCl, 1 mM DTT, 20 mM sodium molybdate, 15 mM MgCl2, 10% glycerol, 1 mM PMSF, the protease inhibitors aprotinin, leupeptin, and pepstatin A, and the phosphatase inhibitors okadaic acid (1 μM), Na2MoO4 (10 mM), Na3VO4 (0.1 mM), and NaF (5 mM). Samples were frozen in liquid nitrogen and stored at −70°C. Extracts, 10 μg each, as determined with the Bio-Rad (Richmond, CA) Bradford reagent, were boiled in SDS sample buffer for 5 min and loaded onto 7.5% SDS-polyacrylamide gels.
GST Pull-Down and Immunoprecipitation Experiments
GST pull-down experiments were performed as follows. Yeast cells (strain RMY326 with plasmids pYes/Ste11ΔN.GST or p2U/GST-2) were washed once with water containing 1 mM DTT and 1 mM PMSF and once with TEG (25 mM Tris-HCl pH 7.4, 15 mM EGTA, 10% glycerol, 1 mM DTT, 1 mM PMSF, 3 μg/ml chymostatin, 1.5 μg/ml pepstatin A, 0.75 μg/ml leupeptin, 3.8 μg/ml antipain) containing 150 mM NaCl. Cell pellets were then resuspended in a small volume of the same buffer and broken with glass beads by two 30-s pulses at maximum speed in a Mini-BeadBeater-8 (Biospec Products, Bartlesville, OK) at 4°C. After centrifugation at 15,000 rpm in a table top centrifuge at 4°C, the supernatant was quantitated and adjusted to 0.1% Triton X-100. Glutathione-sepharose beads (Pharmacia, Piscataway, NJ) were added to the extracts, tumbled for 30–45 min at 4°C, washed three times with TEG containing 150 mM NaCl, 0.1% Triton X-100 and twice with TEG with 0.1% Triton X-100. Bound proteins were eluted with 7.5 mM reduced glutathione in 50 mM Tris-HCl, pH 8.0, and concentrated by trichloroacetic acid (TCA) precipitation, resuspended in SDS sample buffer, and loaded onto 10% SDS-polyacrylamide gels.
Coimmunoprecipitation experiments using the FLAG tag were done as follows. Extracts from strains HH1a-p2TG/flag.Hsp82wt and HH1a-p2G/hsp82wt with and without plasmid pYES/HA-Ste11 were prepared as described above for the GST pull-down experiments except that the buffer was 10 mM Tris-HCl, pH 7.5, 50 mM NaCl, 1 mM DTT, 10 mM sodium molybdate, 1 mM EDTA, 10% glycerol, 1 mM PMSF, 3 μg/ml chymostatin, 1.5 μg/ml pepstatin A, 0.75 μg/ml leupeptin, 3.8 μg/ml antipain. After adjusting the extracts to 0.1% Triton X-100, they were incubated at 4°C with the anti-FLAG monoclonal antibody M2 (Eastman Kodak, Rochester, NY) for 2 h followed by 1 h with Protein G-sepharose (Pharmacia). Immunoprecipitates were washed four times for 10 min at 4°C with the extraction buffer containing 0.1% Triton X-100, solubilized in SDS sample buffer, and loaded onto 10% SDS-polyacrylamide gels. The same protocol was used for immunoprecipitation by a Ste11-specific rabbit polyclonal antiserum (Cairns et al., 1992) of endogenous Ste11 from 0.5 mg of extracts from strains HH1a-p2G/Hsp82wt, HH1a-p2G/Hsp82 G313N, and HH1a-p2G/hHsp90β.
Western Blot Experiments
After transfer of proteins from SDS-polyacrylamide gels to nitrocellulose membranes, the membranes were blocked with Tris-buffered saline, 0.05% Tween-20 (TBST) containing 5% (wt/vol) milk powder and probed with appropriate antibodies in TBST + milk powder at room temperature for 1 h. Mouse anti-GST (Santa Cruz Biotechnology, Santa Cruz, CA), anti-HA (a gift from K. Matter; for references, see Daro et al., 1996), and anti-FLAG (Kodak) monoclonal antibodies, chicken anti-Hsp82 antibodies (Louvion et al., 1996), rabbit polyclonal anti-Hsp82 antiserum (a gift from S. Lindquist), and rabbit polyclonal anti-Ste11 antiserum (Cairns et al., 1992) were diluted 1:1000, 1:100, and to 10 μg/ml, 1:1000, 1:400, and 1:1000, respectively. Membranes were washed three times for 10 min with TBST. The secondary antibodies were alkaline phosphatase-conjugated goat anti-rabbit (Bio-Rad) or anti-chicken (Promega, Madison, WI), horseradish peroxidase-conjugated anti-mouse (Cappel, Cochranville, PA). They were used in TBST + milk powder at room temperature for 1 h. After three washes with TBST, the blots were developed either with the NBT/BCIP reagent for alkaline phosphatase or with the enhanced chemiluminescence reagent (Amersham, Arlington Heights, IL) for horseradish peroxidase.
RESULTS
HSP90 Mutations Interfere with Pheromone-induced Cell Cycle Arrest and Activation of FUS1 Promoter
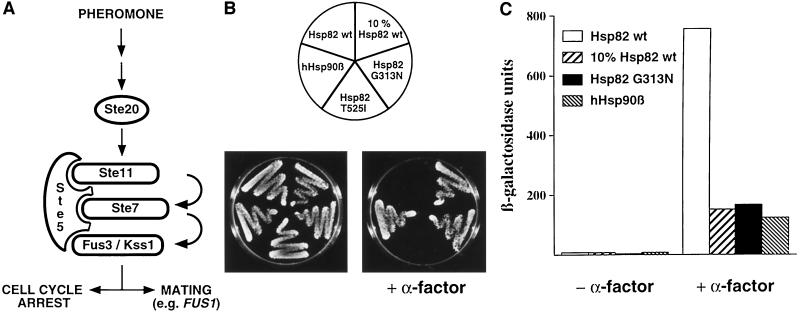
A large variety of HSP90 mutants have been described that complement yeast strains carrying disruptions of the essential chromosomal HSP90 genes, HSP82 and HSC82 (Borkovich et al., 1989; Picard et al., 1990; Bohen and Yamamoto, 1993; Kimura et al., 1994; Minami et al., 1994; Bohen, 1995; Nathan and Lindquist, 1995; Palmer et al., 1995; Louvion et al., 1996). We examined pheromone signaling (Figure 1A) in three types of mutant strains: a strain with only 10% of the normal levels of Hsp82, a strain with human Hsp90β (hHsp90β; hereafter considered a Hsp90 mutant for yeast), and strains expressing specific Hsp82 point mutants. The latter had been found in a screen for defective steroid receptor signaling in yeast. The point mutants T525I and G313N are temperature sensitive for viability and show an impaired hormonal response of glucocorticoid, estrogen, progesterone, and mineralocorticoid receptors (Bohen and Yamamoto, 1993; Bohen, 1995). Hsp82 T525I and Hsp82 G313N are expressed at similar levels as the wild-type Hsp82 (Bohen and Yamamoto, 1993; Bohen, 1995) (see also Figure 4B). We first tested the different mutant strains for their ability to arrest growth in response to the mating pheromone α-factor. As shown in Figure 1B, low levels of Hsp82, point mutant Hsp82 G313N, and hHsp90β are not able to promote a substantial activation of the pheromone pathway as demonstrated by a poor growth arrest in the presence of pheromone. Interestingly, the point mutation T525I discriminates between two different functions of Hsp90, the pheromone and the steroid-signaling pathways being functional and defective, respectively. The other Hsp90 isoform of yeast, Hsc82, as well as the Trypanosoma cruzi Hsp83, which we have previously shown to complement defective yeast strains (Palmer et al., 1995), are also able to support pheromone signaling (our unpublished results).
Figure 1.
Hsp90 is required for pheromone signaling. (A) Schematic representation of the pheromone pathway of budding yeast. Protein kinases Ste11, Ste7, and Fus3/Kss1 are the yeast equivalents of MEKK, MEK, and MAPK, respectively, and are held together by Ste5. Pheromone signaling results in the activation of Ste12 and Far1. The transcription factor Ste12 controls the expression of mating genes, whereas Far1 inhibits G1 cyclins and thereby effects a cell cycle arrest. (B) Yeast strains with HSP90 mutations fail to respond to α-factor. Strains expressing the indicated Hsp90 derivatives were streaked onto YEPD plates with or without 5 μM α-factor. The plates were photographed after 3 d of incubation at 30°C. (C) Induction of the FUS1-LacZ reporter gene by α-factor is strongly reduced in strains with HSP90 mutations. Strains expressing the indicated Hsp90 derivatives were tranformed with the FUS1-LacZ reporter construct. β-Galactosidase expression was quantitated after exposure to α-factor for 2 h.
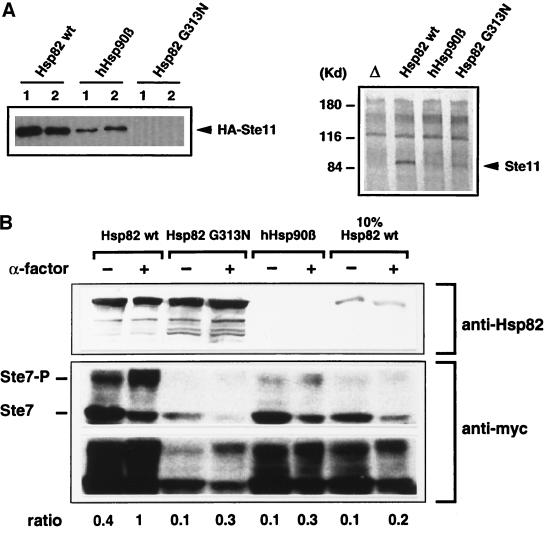
Figure 4.
HSP90 mutations affect the levels of Ste11 and Ste7, and Ste7 hyperphosphorylation. (A) Ste11 protein levels are reduced in Hsp90 mutant strains. Left panel, HA-epitope–tagged Ste11 was overexpressed in Hsp90 mutant strains as indicated; equal amounts of “rapid protein extracts” of two colonies each (1 and 2) were immunoblotted with anti-HA antibodies. Right panel, endogenous wild-type Ste11 was concentrated by immunoprecipitation and immunoblotted with a Ste11-specific antiserum; lane Δ, extracts from a Δste11 control strain. (B) Phosphorylation of Ste7 is reduced by HSP90 mutations. Strains expressing Ste7 containing the myc epitope were exposed for 2 h to α-factor where indicated. Extracts were analyzed by an immunoblot assay using a rabbit polyclonal antibody (a gift from S. Lindquist), which only recognizes the yeast Hsp82 (top panel), and the mouse monoclonal antibody 9E10 against the myc epitope (bottom panel). Two different exposures of the latter immunoblot are presented. The position of the hyperphosphorylated Ste7 (Ste7-P) is indicated. The ratios of hyperphosphorylated Ste7 (Ste7-P) over unphosphorylated Ste7 of all samples were standardized on the ratio obtained in the presence of α-factor with the strain expressing wild-type Hsp82.
The activation of the pheromone pathway also results in the induction of proteins required for cellular and nuclear fusion (mating). To test the Hsp90 requirement in the pheromone-dependent transactivation of mating genes such as FUS1, we assessed the induction of a FUS1-LacZ reporter gene (Trueheart et al., 1987) upon treatment of cells with α-factor. The results shown in Figure 1C indicate that, similarly to what was observed in the case of the G1 arrest, the activation of FUS1-LacZ in response to α-factor is strongly reduced in Hsp90 mutant strains compared with a strain with wild-type Hsp82.
Basal Activity of the Pheromone-signaling Pathway Also Depends on Hsp90
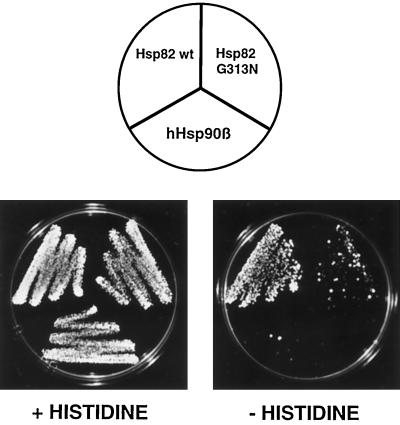
The pheromone pathway exhibits low activity even in the absence of pheromones (Hagen et al., 1991). To determine whether Hsp90 is also required for this basal activity, we examined the activity of a more sensitive reporter gene, FUS1-HIS3, in a his3− strain. Any disruption in the pheromone signaling pathway, such as the complete absence of a component, abrogates the basal activity and prevents growth on medium lacking histidine (Stevenson et al., 1992). As shown in Figure 2, growth on selective medium is severely impaired for Hsp82 G313N and hHsp90β strains when compared with a strain with wild-type Hsp82. In this assay, the hHsp90β strain is reproducibly the most defective. These results indicate that Hsp90 is necessary for both the induced and the basal activity of the pheromone-signaling pathway.
Figure 2.
The basal activity of the pheromone-signaling pathway depends on Hsp90. Strains expressing wild-type (DP122) or mutant Hsp90 (DP123 and DP124) and carrying the FUS1-HIS3 reporter gene were grown on indicator plates containing or lacking histidine. The strains are all derivatives of strain DP121. The plates were photographed after 3 d of incubation at 30°C.
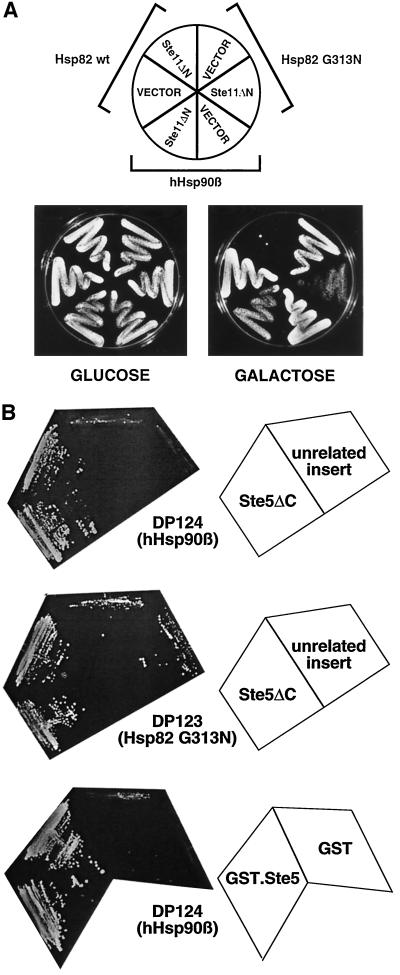
Hsp90 Mutants Block Signaling by Constitutive Ste11
In a first attempt toward determining the step(s) of the pheromone pathway (Figure 1A) that is dependent on Hsp90, we assayed growth arrest induced by a constitutively active Ste11 mutant. It has been shown that the deletion of the amino-terminal regulatory domain of Ste11 (Ste11ΔN) results in constitutive activation of this kinase and in pheromone-independent induction of the mating pathway (Cairns et al., 1992). We constructed such a dominant STE11 and placed it under the control of the conditional GAL1 promoter. When the expression is induced by growth on galactose, only the strain with wild-type Hsp82 exhibits a complete growth arrest (Figure 3A). Strains with either Hsp82 G313N or hHsp90β fail to be fully growth arrested. The same pattern was observed with equivalent strains of the opposite mating type (MATα) (our unpublished results). Thus, these experiments showed that the requirement for Hsp90 is independent of mating type and possibly at the level of Ste11 or downstream of it.
Figure 3.
Hsp90 is required for signaling beyond Ste11 at the level of the MAPK module. (A) Signaling by the constitutively active Ste11ΔN mutant is blocked by HSP90 mutations. Ste11ΔN is conditionally expressed under the control of the GAL1 promoter. Transformants containing the expression plasmid encoding Ste11ΔN (pYES/Ste11ΔN) or the empty plasmid pYES 2.0 (VECTOR) were precultured in selective medium containing glucose as a carbon source and streaked onto repressing (glucose) and inducing(galactose) plates and incubated for 4 d. (B) Ste5 suppresses the signaling defect of Hsp90 mutants. Plasmids encoding C-terminally–truncated Ste5 (Ste5ΔC) and GST fused to full-length Ste5 (GST.Ste5) were introduced into strains carrying the FUS1-HIS3 reporter gene and expressing hHsp90β (strain DP124) or the Hsp82 mutant G313N (strain DP123). The negative controls for Ste5ΔC and GST.Ste5 were another clone from the same library with an unrelated insert and a plasmid expressing GST alone, respectively. Two colonies each were streaked out on indicator plates lacking histidine. The plates were photographed after 3 d of incubation at 30°C.
Ste5 Overexpression Suppresses the Signaling Defect
We performed a screen for high-copy suppressors of the signaling defect of Hsp90 mutant strains. Using the hHsp90β strain with the FUS1-HIS3 reporter, we selected suppressors that allow growth on plates lacking histidine in the absence of pheromone and screened them further for a restored sensitivity to α-factor. In a limited screen with a yeast genomic library in a high-copy vector, only one clone met the two criteria. Sequencing revealed that its genomic insert contains the STE5 gene. It starts 1020 bp upstream of the initiator codon ATG and presumably contains the complete STE5 promoter. At the 3′-end the STE5 sequence is truncated at codon 801 (of 917). The isolated plasmid, denoted Ste5ΔC, thus encodes the first 800 amino acids of Ste5 fused to 15 unrelated amino acids at the C terminus. Further experiments showed that Ste5ΔC is also able to suppress the HSP82 mutation G313N. Figure 3B shows the growth assays on plates lacking histidine and also illustrates that full-length Ste5, as a fusion protein with GST, retains suppressor activity. These data corroborate the tentative conclusion that Hsp90 may be required at the level of the MAPK module consisting of the kinases Ste11, Ste7, and Fus3 that are tethered together by Ste5 (reviewed by Elion, 1995; Leberer et al., 1997).
Ste11 Protein Levels Are Reduced
Certain client proteins of Hsp90, such as Raf-1, the glucocorticoid receptor, or luciferase, appear more susceptible to degradation when interaction with Hsp90 is blocked/altered pharmacologically (Schulte et al., 1995–1997; Schneider et al., 1996; Whitesell and Cook, 1996; Czar et al., 1997; Segnitz and Gehring, 1997; Stancato et al., 1997). We therefore examined the accumulation of Ste11 in Hsp90 mutant strains. An epitope-tagged version of Ste11 was overexpressed under the control of the inducible GAL1 promoter and revealed by immunoblotting (Figure 4A, left panel). In strains with hHsp90β or Hsp82 G313N, Ste11 levels were severely reduced. In the Hsp82 G313N strain Ste11 levels were at the detection limit. At this point we speculated that the levels of the endogenous Ste11, which is difficult to detect, might mirror this pattern. To explore this possibility, we concentrated endogenous wild-type Ste11 by immunoprecipitation with a Ste11-specific antiserum and displayed it by immunoblotting with the same antiserum (Figure 4A, right panel). Despite a relatively high background, the identity of the Ste11 band could be confirmed unambiguously using an extract from a ste11− strain as a control sample (Figure 4A, lane Δ). As in the case of the overexpressed Ste11, accumulation of endogenous Ste11 is reduced in both mutant strains although Hsp82 G313N appears to have a less severe effect on the endogenous than on the overexpressed protein. Thus, reduced levels of Ste11 could, at least in part, explain the functional defects of the pheromone pathway in these strains.
Basal and Induced Phosphorylation of Ste7 Is Reduced
The direct target of the Ste11 kinase is the kinase Ste7 (Figure 1A). Upon exposure to pheromone, Ste7 is activated by phosphorylation by Ste11 and becomes hyperphosphorylated in the presence of Fus3/Kss1 (Zhou et al., 1993; Neiman and Herskowitz, 1994). As shown in Figure 4B, the degree of hyperphosphorylation of Ste7 as well as Ste7 protein levels is strongly reduced in mutant strains. The latter is particularly true for Hsp82 G313N. When compared with the wild-type strain, all the mutant strains show a three- to fivefold reduced hyperphosphorylation of Ste7 both in the absence (basal activity) and in the presence (induced activity) of pheromone. This experiment indicates that Hsp90 function is essential both for Ste7 accumulation and for efficient basal and induced phosphorylation of Ste7.
Ste11 Forms Complexes with Hsp90
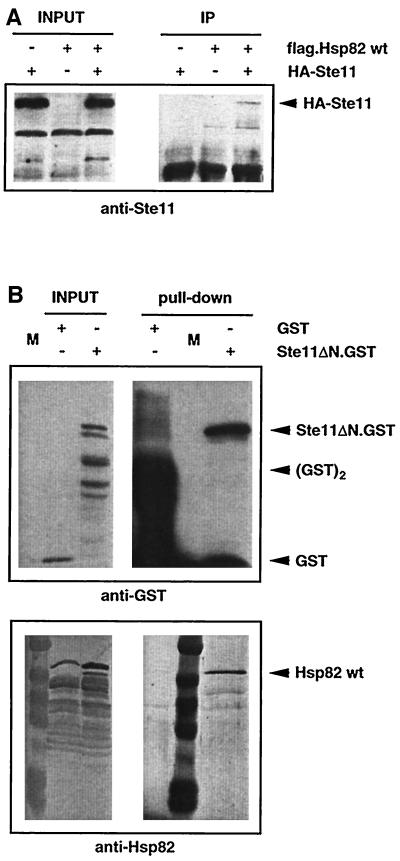
Several experiments described so far suggested that Hsp90 might interact with components of the MAPK module and Ste11 in particular. We performed coprecipitation experiments to examine this issue. Figure 5A shows that HA epitope-tagged Ste11 is specifically coprecipitated with FLAG-tagged Hsp82. The association of Ste11 and yeast Hsp90 (Hsp82) was confirmed by a GST pull-down experiment. GST alone or GST fused to the constitutive Ste11ΔN (Ste11ΔN.GST) was inducibly expressed under the GAL1 promoter in a wild-type strain. While wild-type Hsp82 (and Hsc82) does not associate with GST alone, it specifically coprecipitates with Ste11ΔN.GST (Figure 5B). These results establish that Ste11 exists in complexes with Hsp90 and that the regulatory N-terminal domain of Ste11 is dispensable for this interaction.
Figure 5.
Ste11 forms complexes with Hsp90. (A) Immunoprecipitation experiment showing association of HA-tagged Ste11 with FLAG-tagged Hsp82. INPUT and IP designate equal amounts of total extract and immunoprecipitates, respectively (exposure times are not the same). Note that isogenic strains were used expressing Hsp82 with or without the FLAG tag. HA-Ste11 was revealed by immunoblotting with an anti-Ste11 antiserum. (B) GST pull-down experiment showing association of Ste11ΔN.GST with endogenous Hsp90 (Hsp82/Hsc82). INPUT and “Pull-down” designate equal amounts of total extract and proteins purified with glutathione-sepharose beads, respectively (exposure times are not the same). GST and Ste11ΔN.GST and Hsp82/Hsc82 were revealed by probing the same blot with anti-GST and chicken anti-Hsp82 antibodies, respectively. Note that unfused GST binds the affinity matrix more efficiently, resulting in very strong GST monomer and dimer bands.
Differential Temperature Sensitivity of Hsp90 Mutants
Surprisingly, the Hsp82 requirement for pheromone signaling exhibits a temperature dependence. The mutant phenotype of Hsp82 G313N and hHsp90β strains was not apparent in all assays at the lower temperature of 21°C (Table 1). This is particularly striking for G313N whose response in all our assays is only slightly reduced compared with wild-type Hsp82 at 21°C. In contrast, low amounts of wild-type Hsp82 are unable to support signaling in response to α-factor even at the lower temperature. Similarly, the basal and α-factor–induced activities of the pheromone pathway are defective in strains with hHsp90β at both temperatures. Interestingly, a full growth arrest is observed at 21°C with a hHsp90β strain when the pheromone pathway is activated with the constitutive Ste11ΔN. This suggests that Hsp90 function might be required differentially both “upstream” and “downstream” of Ste11. At low temperature, hHsp90β appears to be able to fulfill the downstream, but not the upstream requirement. Since none of the mutations are able to block the Ste11ΔN-induced cell cycle arrest at the lower temperature, we cannot formally rule out the possibility that Hsp90 function is not required at all for this particular response. However, this seems unlikely in view of the striking signaling defects at 30°C and may be due to the vast overexpression of Ste11ΔN in this assay and initiation of signaling at an intermediate level.
Table 1.
Summary of the phenotypes of the Hsp90 mutants
| Hsp82 mutants | G1 arrest (α-factor)
|
G1 arrest (Ste 11ΔN)
|
FUS1-LacZ expression
|
FUS1-HIS3 expression
|
||||
|---|---|---|---|---|---|---|---|---|
| 30°C | 21°C | 30°C | 21°C | 30°C | 21°C | 30°C | 21°C | |
| Hsp82 wt | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ |
| 10% Hsp82 wt | − | − | NA | NA | +/− | +/− | ND | ND |
| Hsp82 G313N | − | ++ | +/− | +++ | +/− | ++ | − | ++ |
| Hsp82 T525l | +++ | +++ | +++ | +++ | ND | ND | ND | ND |
| hHsp90β | − | − | +/− | +++ | +/− | +/− | − | − |
Note that this table also includes all the results presented in Figures 1, B and C, 2, and 3A, which were obtained by culturing cells at 30°C. ND, not done; NA, not applicable. +++, ++ and +/− indicate full, partial, and weak responses, respectively.
DISCUSSION
Using a series of Hsp90 mutants we have demonstrated that pheromone signaling through the MAPK cascade depends on Hsp90 function. Hsp90 is required both for the basal activity of this pathway in the absence of pheromone and for efficient induction upon exposure to pheromone. A combination of genetic and biochemical experiments pinpoints Ste11, a yeast equivalent of Raf, as a target of Hsp90. Since mammalian Raf-1 can substitute for Ste11 under certain circumstances (Freed et al., 1994; Irie et al., 1994), our results also set the stage for using yeast genetics to investigate the role of Hsp90 for Raf function and for mammalian MAPK signaling.
Ste11 Depends on Hsp90 Function
Our results support the conclusion that pheromone signaling depends on Hsp90 at the level of Ste11: 1) The constitutive Ste11 mutant (Ste11ΔN) fails to elicit a complete cell cycle arrest (at 30°C) in Hsp90 mutant strains; 2) The levels of both endogenous and overexpressed Ste11 are reduced in mutant strains; 3) The basal and induced phosphorylation of the Ste11 substrate Ste7 are reduced in mutant strains; 4) Ste11 and Hsp90 (Hsp82) are found in a complex.
The reduction of Ste11 protein levels are an indication that Hsp90 may be required to ensure the stability of Ste11. Since the plasmids that we used for overexpression of Ste11 contained exclusively the STE11 coding body, the rate of synthesis is likely to be similar. This leads to the tentative conclusion that it is the turnover of Ste11 that is increased in Hsp90 mutant strains. Whether the destabilization of Ste11 is due to misfolding and/or a failure to form complexes with other factors remains to be determined. While the effects on Ste11 protein levels could also be indirect, the finding that Hsp90 and Ste11 form complexes suggests that it is the altered nature of these complexes in Hsp90 mutant strains that leads to enhanced degradation of Ste11. The low levels of Ste11 in these strains have so far precluded experiments to determine whether Hsp90 mutants form complexes with Ste11 at all. In vitro experiments with purified components might allow assessment of whether the Ste11-Hsp90 interaction is direct and how it is affected by alterations of Hsp90. Further analyses will also have to establish the stoichiometry of the complex and the proportion of Ste11 that is associated with Hsp90 at any given time. Interestingly, the effects of mutating HSP90 in yeast are mirrored by pharmacological experiments with the Hsp90 “drug” geldanamycin (or herbimycin A, another ansamycin) in vertebrate cells. Raf-1 is degraded when cells are treated with this compound (Schulte et al., 1995–1997; Schneider et al., 1996; Stancato et al., 1997). Similar effects have been reported for the glucocorticoid receptor, another Hsp90 substrate (Whitesell and Cook, 1996; Czar et al., 1997; Segnitz and Gehring, 1997). Although accumulation of Raf was apparently not affected in Drosophila strains with HSP83 mutations (van der Straten et al., 1997), it should be pointed out that the severity of the effect also depends on the mutation in our system. Recently, Errede and her collaborators have obtained results that support our conclusions. They could notably demonstrate with a temperature-sensitive Hsp82 mutant (Nathan and Lindquist, 1995) that the accumulation of newly synthesized Ste11 depends on continuous Hsp90 function (Buehrer, Rhodes, Rutherford, and Errede, unpublished data).
The reduced accumulation of Ste11 (and possibly Ste7) might be sufficient to account for the mutant phenotype. Since it is technically difficult to measure the specific activity of Ste11, we cannot exclude that Ste11 also requires Hsp90 to reach its full enzymatic activity. The residual number of Ste11 molecules in Hsp90 mutant strains might well be sufficient, but they may have a lower specific activity. In the case of geldanamycin-treated vertebrate cells, specific activity of Raf appears to remain unchanged (Stancato et al., 1997) whereas in Drosophila its specific activity appeared to be affected by HSP83 mutations (van der Straten et al., 1997).
The Hsp90 mutant strains that we have tested are not completely defective in Ste11 activity. Unlike ste11 deletion strains, they are able to form shmoos in response to pheromone, and they can mate albeit with reduced efficiency (our unpublished results). The hyperphosphorylation of Ste7 that occurs at a lower level even in Hsp90 mutant strains further corroborates that there is residual Ste11 activity. This is either due to a pathway that allows Ste11 maturation/stabilization to proceed partially in an Hsp90-independent manner or to residual activity of our panel of Hsp90 mutants. Indeed, Hsp90 mutants that are both viable and completely defective for this specific function may be difficult to find. Along with the fact that there are two genes for Hsp90 in S. cerevisiae, this residual Ste11 activity probably explains why HSP90 was never found in screens for sterile mutants.
Is Ste11 the Only Substrate of Hsp90 in the Pheromone-signaling Pathway?
Both biochemical evidence and results obtained with the yeast two-hybrid system have led to the view that Ste11, Ste7, Fus3/Kss1, and other components of the pheromone pathway are all tethered together by Ste5. Ste5 may serve as a scaffold to maintain the different kinases and their substrates in a macromolecular signal transduction complex, thereby ensuring specificity and efficiency (for reviews, see Elion, 1995; Leberer et al., 1997). This illustrates that the notion of a linear signal transduction from upstream to downstream components, as derived from genetic epistasis experiments, is too simplistic. Moreover, it does not take into account that additional factors such as molecular chaperones could be required for the maturation of the individual components and/or the multiprotein complex. Two linked hypotheses are worth considering in this context: 1) Hsp90 chaperones the dynamic assembly of this multiprotein signaling complex; 2) Hsp90 is required for the maturation/stabilization of additional signaling molecules. Note that Hsp90 does not have to be a stable component of these complexes; it might only transiently interact with Ste11 and/or other proteins.
To address the first hypothesis the tools have yet to be developed. Using the yeast two-hybrid assay that relies on interactions of chimeras in the nucleus, we have not seen any differences in Hsp90 mutant strains for the interactions of Ste5 with Ste11 or Ste7 (our unpublished results). However, it will ultimately be necessary to characterize the complex formed of the endogenous wild-type proteins, a technically daunting task.
Regarding the second hypothesis, the reduced Ste7 levels are compatible, but not more, with an interaction of Ste7 with Hsp90. The differential behavior of certain Hsp90 mutants, notably hHsp90β, at different temperatures in different assays (see Table 1) might also support such an assumption. While hHsp90β allows signaling by the constitutive Ste11ΔN at low temperature, it fails to allow pheromone to signal through the complete pathway. Interestingly, Ste5 overexpression suppresses the signaling defect of Hsp90 mutant strains, but only biochemical experiments will be able to elucidate how this increases the efficiency of the signaling complex. Taking these observations as guidelines, the interaction of Hsp90 with signaling molecules both upstream and downstream of Ste11 as well as Ste5 will have to be examined directly.
Hsp90 Requirement in Other MAPK Pathways
Other MAPK-signaling pathways in yeast may also depend on Hsp90. While the cell wall integrity pathway does not appear to be affected in our Hsp90 mutant strains (our unpublished results), other pathways await examination. This will be particularly interesting for the three other pathways that are known to share Ste11: one of the two osmoregulatory pathways (Posas and Saito, 1997), the invasive growth response of haploid cells, and pseudohyphal development of diploids (see Herskowitz, 1995; Levin and Errede, 1995; Schultz et al., 1995). In this context it is noteworthy that the growth arrest/“toxicity” induced by Ste11ΔN appears to be due to its functions in both the pheromone and the high osmolarity response pathways (Posas and Saito, 1997). Since Hsp90 mutant strains are at least partially refractory to the Ste11ΔN toxicity, we speculate that Hsp90 may be required for Ste11 function in both pathways.
Genetic Dissection of Different Hsp90 Functions
Previous studies had demonstrated that it is possible to selectively abolish specific dispensable functions of Hsp90 without compromising its ability to ensure viability in yeast; specifically, a variety of HSP82 mutations result in a defect in the regulation of steroid receptors or v-Src or folding of p53 in yeast (Picard et al., 1990; Bohen and Yamamoto, 1993; Xu and Lindquist, 1993; Bohen, 1995; Nathan and Lindquist, 1995; Blagosklonny et al., 1996; Fang et al., 1996; Nathan et al., 1997). We have now considerably extended this theme by showing that even subtle point mutations can discriminate between the Hsp90 requirements in two different signaling pathways. Some mutants, like the Hsp82 point mutant G313N, are defective in both steroid receptor and pheromone signaling. Another point mutant, T525I, is only defective in steroid receptor signaling while the converse is true for human Hsp90 (this article and our unpublished results). Moreover, G313N has a different temperature sensitivity for several Hsp90 functions: at room temperature, only hormone binding of steroid receptors is defective (Bohen, 1995) whereas viability (Bohen and Yamamoto, 1993) and pheromone signaling are only lost upon increasing the temperature to 37°C and 30°C, respectively. A deletion analysis of HSP82 has proven of limited use in assigning specific functions to individual domains of Hsp90 (Louvion et al., 1996). Only two regions, the eukaryote-specific N-terminal charged domain and the C-terminal conserved pentapetide, could be deleted without affecting viability. These two portions of Hsp82 are also dispensable for Hsp90 function in pheromone signaling (Louvion et al., 1996). By ensuring viability with human Hsp90 (hHsp90β), which cannot promote pheromone signaling, it might nevertheless be possible to map the domains of Hsp82 that are specifically required for its function in pheromone signaling. In such a system, even coexpressed Hsp82 mutants, which fail to provide the viability function, might be able to restore pheromone signaling. Additional insights could be gained by examining a series of chimeras between yeast Hsp82 and human Hsp90β.
ACKNOWLEDGMENTS
We thank S.P. Bohen and K.R. Yamamoto, B. Cairns, M. Collart, E.A. Elion, B. Errede, G.R. Fink, T. Kreis, S. Lindquist, K. Matter, R. Movva, G. Sprague, and D.O. Toft for plasmids, strains, antibodies, and other reagents. We acknowledge the sequencing services of S. Antonorakis and of the Department of Molecular Biology. We are grateful to M. Strubin and J. Geiselmann for critical comments on an early version of the manuscript. We thank B. Errede for her thoughtful comments and for communicating unpublished results. This work was supported by the Swiss National Science Foundation and the Canton de Genève.
Abbreviations used:
- HBD
hormone-binding domain
- Hsp
heat-shock protein
REFERENCES
- Aligue R, Akhavan-Niak H, Russell P. A role for Hsp90 in cell cycle control: Wee1 tyrosine kinase activity requires interaction with Hsp90. EMBO J. 1994;13:6099–6106. doi: 10.1002/j.1460-2075.1994.tb06956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonsson C, Whitelaw ML, McGuire J, Gustafsson J-Å, Poellinger L. Distinct roles of the molecular chaperone hsp90 in modulating dioxin receptor function via the basic helix-loop-helix and PAS domains. Mol Cell Biol. 1995;15:756–765. doi: 10.1128/mcb.15.2.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamberger CM, Wald M, Bamberger AM, Schulte HM. Inhibition of mineralocorticoid and glucocorticoid receptor function by the heat shock protein 90-binding agent geldanamycin. Mol Cell Endocrinol. 1997;131:233–240. doi: 10.1016/s0303-7207(97)00115-9. [DOI] [PubMed] [Google Scholar]
- Bardwell JCA, Craig EA. Ancient heat shock gene is dispensable. J Bacteriol. 1988;7:2977–2983. doi: 10.1128/jb.170.7.2977-2983.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagosklonny MV, Toretsky J, Bohen S, Neckers L. Mutant conformation of p53 translated in vitro or in vivo requires functional HSP90. Proc Natl Acad Sci USA. 1996;93:8379–8383. doi: 10.1073/pnas.93.16.8379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohen SP. Hsp90 mutants disrupt glucocorticoid receptor ligand binding and destabilize aporeceptor complexes. J Biol Chem. 1995;270:29433–29438. doi: 10.1074/jbc.270.49.29433. [DOI] [PubMed] [Google Scholar]
- Bohen SP, Yamamoto KR. Isolation of Hsp90 mutants by screening for decreased steroid receptor function. Proc Natl Acad Sci USA. 1993;90:11424–11428. doi: 10.1073/pnas.90.23.11424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borkovich KA, Farrelly FW, Finkelstein DB, Taulien J, Lindquist S. hsp82 is an essential protein that is required in higher concentrations for growth of cells at higher temperatures. Mol Cell Biol. 1989;9:3919–3930. doi: 10.1128/mcb.9.9.3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns BR, Ramer SW, Kornberg RD. Order of action of components in the yeast pheromone response pathway revealed with a dominant allele of the STE11 kinase and the multiple phosphorylation of the STE7 kinase. Genes & Dev. 1992;6:1305–1318. doi: 10.1101/gad.6.7.1305. [DOI] [PubMed] [Google Scholar]
- Carver LA, Jackiw V, Bradfield CA. The 90-kDa heat shock protein is essential for Ah receptor signaling in a yeast expression system. J Biol Chem. 1994;269:30109–30112. [PubMed] [Google Scholar]
- Choi K-Y, Satterberg B, Lyons DM, Elion EA. Ste5 tethers multiple protein kinases in the MAP kinase cascade required for mating in S. cerevisiae. Cell. 1994;78:499–512. doi: 10.1016/0092-8674(94)90427-8. [DOI] [PubMed] [Google Scholar]
- Coumailleau P, Poellinger L, Gustafsson J-Å, Whitelaw ML. Definition of a minimal domain of the dioxin receptor that is associated with Hsp90 and maintains wild type ligand binding affinity and specificity. J Biol Chem. 1995;270:25291–25300. doi: 10.1074/jbc.270.42.25291. [DOI] [PubMed] [Google Scholar]
- Csermely P, Schnaider T, Sôti C, Prohászka Z, Nardai G. The 90 kDa molecular chaperone family: structure, function and clinical applications. Pharmacol & Ther. 1998;79:129–168. doi: 10.1016/s0163-7258(98)00013-8. [DOI] [PubMed] [Google Scholar]
- Cutforth T, Rubin GM. Mutations in Hsp83 and cdc37 impair signaling by the sevenless receptor tyrosine kinase in Drosophila. Cell. 1994;77:1027–1036. doi: 10.1016/0092-8674(94)90442-1. [DOI] [PubMed] [Google Scholar]
- Czar MJ, Galigniana MD, Silverstein AM, Pratt WB. Geldanamycin, a heat shock protein 90-binding benzoquinone ansamycin, inhibits steroid-dependent translocation of the glucocorticoid receptor from the cytoplasm to the nucleus. Biochemistry. 1997;36:7776–7785. doi: 10.1021/bi970648x. [DOI] [PubMed] [Google Scholar]
- Daro E, Van der Sluijs P, Galli T, Mellman I. Rab4 and cellubrevin define different early endosome populations on the pathway of transferrin receptor recycling. Proc Natl Acad Sci USA. 1996;93:9559–9564. doi: 10.1073/pnas.93.18.9559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle HJ, Bishop JM. Torso, a receptor tyrosine kinase required for embryonic pattern formation, shares substrates with the Sevenless and EGF-R pathways in Drosophila. Genes & Dev. 1993;7:633–646. doi: 10.1101/gad.7.4.633. [DOI] [PubMed] [Google Scholar]
- Elion EA. Ste5: a meeting place for MAP kinases and their associates. Trends Cell Biol. 1995;5:322–327. doi: 10.1016/s0962-8924(00)89055-8. [DOI] [PubMed] [Google Scholar]
- Fang Y, Fliss AE, Robins DM, Caplan AJ. Hsp90 regulates androgen receptor hormone binding affinity in vivo. J Biol Chem. 1996;271:28697–28702. doi: 10.1074/jbc.271.45.28697. [DOI] [PubMed] [Google Scholar]
- Freed E, Symons M, Macdonald SG, McCormick F, Ruggieri R. Binding of 14–3-3 proteins to the protein kinase Raf and effects on its activation. Science. 1994;265:1713–1716. doi: 10.1126/science.8085158. [DOI] [PubMed] [Google Scholar]
- Freeman BC, Morimoto RI. The human cytosolic molecular chaperones hsp90, hsp70 (hsc70) and hdj-1 have distinct roles in recognition of a non-native protein and protein refolding. EMBO J. 1996;15:2969–2979. [PMC free article] [PubMed] [Google Scholar]
- Gietz RD, Sugino A. New yeast-Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-base pair restriction sites. Gene. 1988;74:527–534. doi: 10.1016/0378-1119(88)90185-0. [DOI] [PubMed] [Google Scholar]
- Hagen DC, McCaffrey G, Sprague GF., Jr Pheromone response elements are necessary and sufficient for basal and pheromone-induced transcription of the FUS1 gene of Saccharomyces cerevisiae. Mol Cell Biol. 1991;11:2952–2961. doi: 10.1128/mcb.11.6.2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartson SD, Barrett DJ, Burn P, Matts RL. Hsp90-mediated folding of the lymphoid cell kinase p56lck. Biochemistry. 1996;35:13451–13459. doi: 10.1021/bi961332c. [DOI] [PubMed] [Google Scholar]
- Herskowitz I. MAP kinase pathways in yeast: for mating and more. Cell. 1995;80:187–197. doi: 10.1016/0092-8674(95)90402-6. [DOI] [PubMed] [Google Scholar]
- Holley SJ, Yamamoto KR. A role for Hsp90 in retinoid receptor signal transduction. Mol Biol Cell. 1995;6:1833–1842. doi: 10.1091/mbc.6.12.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath A, Riezman H. Rapid protein extraction from Saccharomyces cerevisiae. Yeast. 1994;10:1305–1310. doi: 10.1002/yea.320101007. [DOI] [PubMed] [Google Scholar]
- Irie K, Gotoh Y, Yashar BM, Errede B, Nishida E, Matsumoto K. Stimulatory effects of yeast and mammalian 14–3-3 proteins on the Raf protein kinase. Science. 1994;265:1716–1719. doi: 10.1126/science.8085159. [DOI] [PubMed] [Google Scholar]
- Jakob U, Buchner J. Assisting spontaneity: the role of Hsp90 and small Hsps as molecular chaperones. Trends Biochem Sci. 1994;19:205–211. doi: 10.1016/0968-0004(94)90023-x. [DOI] [PubMed] [Google Scholar]
- Jakob U, Lilie H, Meyer I, Buchner J. Transient interaction of hsp90 with early unfolding intermediates of citrate synthase — implications for heat shock in vivo. J Biol Chem. 1995a;270:7288–7294. doi: 10.1074/jbc.270.13.7288. [DOI] [PubMed] [Google Scholar]
- Jakob U, Meyer I, Bugl H, Andre S, Bardwell JC, Buchner J. Structural organization of procaryotic and eucaryotic Hsp90. Influence of divalent cations on structure and function. J Biol Chem. 1995b;270:14412–14419. doi: 10.1074/jbc.270.24.14412. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Matsumoto S, Yahara I. Temperature-sensitive mutants of hsp82 of the budding yeast Saccharomyces cerevisiae. Mol & Gen Genet. 1994;242:517–527. doi: 10.1007/BF00285275. [DOI] [PubMed] [Google Scholar]
- Leberer E, Thomas DY, Whiteway M. Pheromone signalling and polarized morphogenesis in yeast. Curr Opin Genet Dev. 1997;7:59–66. doi: 10.1016/s0959-437x(97)80110-4. [DOI] [PubMed] [Google Scholar]
- Levin DE, Errede B. The proliferation of MAP kinase signaling pathways in yeast. Curr Opin Cell Biol. 1995;7:197–202. doi: 10.1016/0955-0674(95)80028-x. [DOI] [PubMed] [Google Scholar]
- Louvion J-F, Warth R, Picard D. Two eukaryote-specific regions of Hsp82 are dispensable for its viability and signal transduction functions in yeast. Proc Natl Acad Sci USA. 1996;93:13937–13942. doi: 10.1073/pnas.93.24.13937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovric J, Bischof O, Moelling K. Cell cycle-dependent association of Gag-Mil and hsp90. FEBS Lett. 1994;343:15–21. doi: 10.1016/0014-5793(94)80598-9. [DOI] [PubMed] [Google Scholar]
- McGuire J, Whitelaw ML, Pongratz I, Gustafsson J-Å, Poellinger L. A cellular factor stimulates ligand-dependent release of hsp90 from the basic helix-loop-helix dioxin receptor. Mol Cell Biol. 1994;14:2438–2446. doi: 10.1128/mcb.14.4.2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami Y, Kimura Y, Kawasaki H, Suzuki K, Yahara I. The carboxy-terminal region of mammalian HSP90 is required for its dimerization and function in vivo. Mol Cell Biol. 1994;14:1459–1464. doi: 10.1128/mcb.14.2.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata Y, Yahara I. The 90-kDa heat shock protein, HSP90, binds and protects casein kinase II from self-aggregation and enhances its kinase activity. J Biol Chem. 1992;267:7042–7047. [PubMed] [Google Scholar]
- Nathan DF, Lindquist S. Mutational analysis of Hsp90 function: interactions with a steroid receptor and a protein kinase. Mol Cell Biol. 1995;15:3917–3925. doi: 10.1128/mcb.15.7.3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan DF, Vos MH, Lindquist S. In vivo functions of the Saccharomyces cerevisiae Hsp90 chaperone. Proc Natl Acad Sci USA. 1997;94:12949–12956. doi: 10.1073/pnas.94.24.12949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neiman AM, Herskowitz I. Reconstitution of a yeast protein kinase cascade in vitro: activation of the yeast MEK homologue STE7 by STE11. Proc Natl Acad Sci USA. 1994;91:3398–3402. doi: 10.1073/pnas.91.8.3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer G, Louvion J-F, Tibbetts RS, Engman DM, Picard D. Trypanosoma cruzi heat-shock protein 90 can functionally complement yeast. Mol Biochem Parasitol. 1995;70:199–202. doi: 10.1016/0166-6851(95)00007-n. [DOI] [PubMed] [Google Scholar]
- Picard D. Steroid-binding domains for regulating the functions of heterologous proteins in cis. Trends Cell Biol. 1993;3:278–280. doi: 10.1016/0962-8924(93)90057-8. [DOI] [PubMed] [Google Scholar]
- Picard D. Regulation of protein function through expression of chimaeric proteins. Curr Opin Biotech. 1994;5:511–515. doi: 10.1016/0958-1669(94)90066-3. [DOI] [PubMed] [Google Scholar]
- Picard D. The role of heat shock proteins in the regulation of steroid receptor function. In: Freedman LP, editor. The Molecular Biology of Steroid and Nuclear Hormone Receptors. Boston: Birkhäuser; 1998. pp. 1–18. [Google Scholar]
- Picard D, Khursheed B, Garabedian MJ, Fortin MG, Lindquist S, Yamamoto KR. Reduced levels of hsp90 compromise steroid receptor action in vivo. Nature. 1990;348:166–168. doi: 10.1038/348166a0. [DOI] [PubMed] [Google Scholar]
- Pongratz I, Mason GG, Poellinger L. Dual roles of the 90-kDa heat shock protein hsp90 in modulating functional activities of the dioxin receptor. Evidence that the dioxin receptor functionally belongs to a subclass of nuclear receptors which require hsp90 both for ligand binding activity and repression of intrinsic DNA binding activity. J Biol Chem. 1992;267:13728–13734. [PubMed] [Google Scholar]
- Posas F, Saito H. Osmotic activation of the HOG MAPK pathway via Ste11p MAPKKK: scaffold role of Pbs2p MAPKK. Science. 1997;276:1702–1705. doi: 10.1126/science.276.5319.1702. [DOI] [PubMed] [Google Scholar]
- Pratt WB, Toft DO. Steroid receptor interactions with heat shock protein and immunophilin chaperones. Endocr Rev. 1997;18:306–360. doi: 10.1210/edrv.18.3.0303. [DOI] [PubMed] [Google Scholar]
- Rebbe NF, Ware J, Bertina RM, Modrich P, Stafford DW. Nucleotide sequence of a cDNA for a member of the human 90 kD heat shock protein family. Gene. 1987;53:235–245. doi: 10.1016/0378-1119(87)90012-6. [DOI] [PubMed] [Google Scholar]
- Rhodes N, Connell L, Errede B. STE11 is a protein kinase required for cell-type-specific transcription and signal transduction in yeast. Genes & Dev. 1990;4:1862–1874. doi: 10.1101/gad.4.11.1862. [DOI] [PubMed] [Google Scholar]
- Schneider C, Sepp Lorenzino L, Nimmesgern E, Ouerfelli O, Danishefsky S, Rosen N, Hartl FU. Pharmacologic shifting of a balance between protein refolding and degradation mediated by Hsp90. Proc Natl Acad Sci USA. 1996;93:14536–14541. doi: 10.1073/pnas.93.25.14536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuh S, Yonemoto W, Brugge J, Bauer VJ, Riehl RM, Sullivan WP, Toft DO. A 90,000-dalton binding protein common to both steroid receptors and the Rous sarcoma virus transforming protein Pp 60v-src. J Biol Chem. 1985;260:14292–14296. [PubMed] [Google Scholar]
- Schulte TW, An WG, Neckers LM. Geldanamycin-induced destabilization of Raf-1 involves the proteasome. Biochem Biophys Res Commun. 1997;239:655–659. doi: 10.1006/bbrc.1997.7527. [DOI] [PubMed] [Google Scholar]
- Schulte TW, Blagosklonny MV, Ingui C, Neckers L. Disruption of the Raf-1-Hsp90 molecular complex results in destabilization of Raf-1 and loss of Raf-1-Ras association. J Biol Chem. 1995;270:24585–24588. doi: 10.1074/jbc.270.41.24585. [DOI] [PubMed] [Google Scholar]
- Schulte TW, Blagosklonny MV, Romanova L, Mushinski JF, Monia BP, Johnston JF, Nguyen P, Trepel J, Neckers LM. Destabilization of Raf-1 by geldanamycin leads to disruption of the Raf-1-MEK-mitogen-activated protein kinase signalling pathway. Mol Cell Biol. 1996;16:5839–5845. doi: 10.1128/mcb.16.10.5839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz J, Ferguson B, Sprague GF., Jr Signal transduction and growth control in yeast. Curr Opin Genet Dev. 1995;5:31–37. doi: 10.1016/s0959-437x(95)90050-0. [DOI] [PubMed] [Google Scholar]
- Segnitz B, Gehring U. The function of steroid hormone receptors is inhibited by the hsp90-specific compound geldanamycin. J Biol Chem. 1997;272:18694–18701. doi: 10.1074/jbc.272.30.18694. [DOI] [PubMed] [Google Scholar]
- Shaknovich R, Shue G, Kohtz DS. Conformational activation of a basic helix-loop-helix protein (MyoD1) by the C-terminal region of murine HSP90 (HSP84) Mol Cell Biol. 1992;12:5059–5068. doi: 10.1128/mcb.12.11.5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shue GL, Kohtz DS. Structural and functional aspects of basic helix-loop-helix protein folding by heat-shock protein-90. J Biol Chem. 1994;269:2707–2711. [PubMed] [Google Scholar]
- Sikorski RS, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DF, Whitesell L, Nair SC, Chen S, Prapapanich V, Rimerman RA. Progesterone receptor structure and function altered by geldanamycin, an hsp90-binding agent. Mol Cell Biol. 1995;15:6804–6812. doi: 10.1128/mcb.15.12.6804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stancato LF, Chow YH, Hutchison KA, Perdew GH, Jove R, Pratt WB. Raf exists in a native heterocomplex with hsp90 and p50 that can be reconstituted in a cell-free system. J Biol Chem. 1993;268:21711–21716. [PubMed] [Google Scholar]
- Stancato LF, Chow YH, Owensgrillo JK, Yem AW, Deibel MR, Jove R, Pratt WB. The native v-Raf·hsp90·p50 heterocomplex contains a novel immunophilin of the FK506 binding class. J Biol Chem. 1994;269:22157–22161. [PubMed] [Google Scholar]
- Stancato LF, Silverstein AM, Owens-Grillo JK, Chow Y-H, Jove R, Pratt WB. The Hsp90-binding antibiotic geldanamycin decreases Raf levels and epidermal growth factor signaling without disrupting formation of signaling complexes or reducing the specific enzymatic activity of Raf kinase. J Biol Chem. 1997;272:4013–4020. doi: 10.1074/jbc.272.7.4013. [DOI] [PubMed] [Google Scholar]
- Stevenson BJ, Rhodes N, Errede B, Sprague GF., Jr Constitutive mutants of the protein kinase Ste11 activate the yeast pheromone response pathway in the absence of the G protein. Genes & Dev. 1992;6:1293–1304. doi: 10.1101/gad.6.7.1293. [DOI] [PubMed] [Google Scholar]
- Takata Y, Imamura T, Iwata M, Usui I, Haruta T, Nandachi N, Ishiki M, Sasaoka T, Kobayashi M. Functional importance of heat shock protein 90 associated with insulin receptor on insulin-stimulated mitogenesis. Biochem Biophys Res Commun. 1997;237:345–347. doi: 10.1006/bbrc.1997.7116. [DOI] [PubMed] [Google Scholar]
- Trueheart J, Boeke JD, Fink GR. Two genes required for cell fusion during yeast conjugation: evidence for a pheromone-induced surface protein. Mol Cell Biol. 1987;7:2316–2328. doi: 10.1128/mcb.7.7.2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Straten A, Rommel C, Dickson B, Hafen E. The heat shock protein 83 (Hsp83) is required for Raf-mediated signalling in Drosophila. EMBO J. 1997;16:1961–1969. doi: 10.1093/emboj/16.8.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warth R, Briand P-A, Picard D. Functional analysis of the yeast 40 kDa cyclophilin Cyp40 and its role for viability and steroid receptor regulation. Biol Chem. 1997;378:381–391. doi: 10.1515/bchm.1997.378.5.381. [DOI] [PubMed] [Google Scholar]
- Wartmann M, Davis RJ. The native structure of the activated Raf protein kinase is a membrane-bound multi-subunit complex. J Biol Chem. 1994;269:6695–6701. [PubMed] [Google Scholar]
- Whitelaw ML, McGuire J, Picard D, Gustafsson J-Å, Poellinger L. Heat shock protein hsp90 regulates dioxin receptor function in vivo. Proc Natl Acad Sci USA. 1995;92:4437–4441. doi: 10.1073/pnas.92.10.4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitesell L, Cook P. Stable and specific binding of heat shock protein 90 by geldanamycin disrupts glucocorticoid receptor function in intact cells. Mol Endocrinol. 1996;10:705–712. doi: 10.1210/mend.10.6.8776730. [DOI] [PubMed] [Google Scholar]
- Whitesell L, Mimnaugh EG, De Costa B, Myers CE, Neckers LM. Inhibition of heat shock protein HSP90-Pp 60v-src heteroprotein complex formation by benzoquinone ansamycins: essential role for stress proteins in oncogenic transformation. Proc Natl Acad Sci USA. 1994;91:8324–8328. doi: 10.1073/pnas.91.18.8324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiech H, Buchner J, Zimmermann R, Jakob U. Hsp90 chaperones protein folding in vitro. Nature. 1992;358:169–170. doi: 10.1038/358169a0. [DOI] [PubMed] [Google Scholar]
- Xu Y, Lindquist S. Heat-shock protein hsp90 governs the activity of Pp 60v-src kinase. Proc Natl Acad Sci USA. 1993;90:7074–7078. doi: 10.1073/pnas.90.15.7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yocum RR, Hanley S, West RW, Jr, Ptashne M. Use of lacZ fusions to delimit regulatory elements of the inducible divergent GAL1-GAL10 promoter in Saccharomyces cerevisiae. Mol Cell Biol. 1984;4:1985–1998. doi: 10.1128/mcb.4.10.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonehara M, Minami Y, Kawata Y, Nagai J, Yahara I. Heat-induced chaperone activity of HSP90. J Biol Chem. 1996;271:2641–2645. doi: 10.1074/jbc.271.5.2641. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Gartner A, Cade R, Ammerer G, Errede B. Pheromone-induced signal transduction in Saccharomyces cerevisiae requires the sequential function of three protein kinases. Mol Cell Biol. 1993;13:2069–2080. doi: 10.1128/mcb.13.4.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]