Abstract
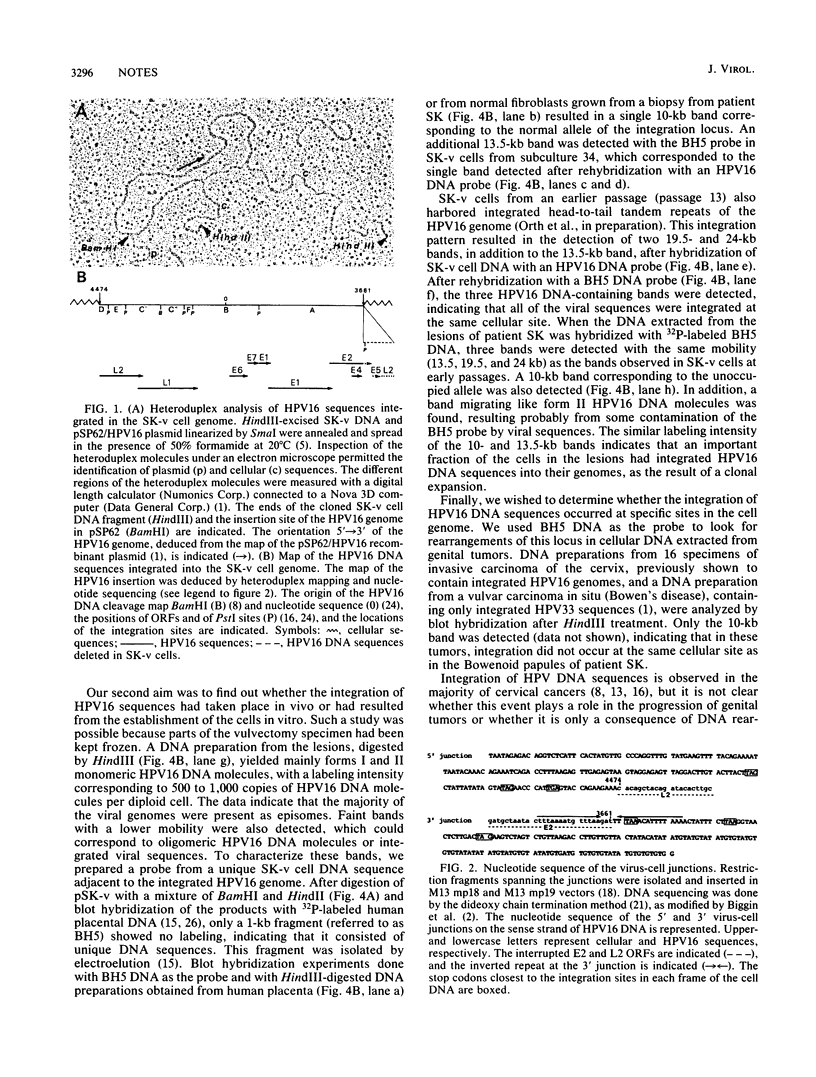
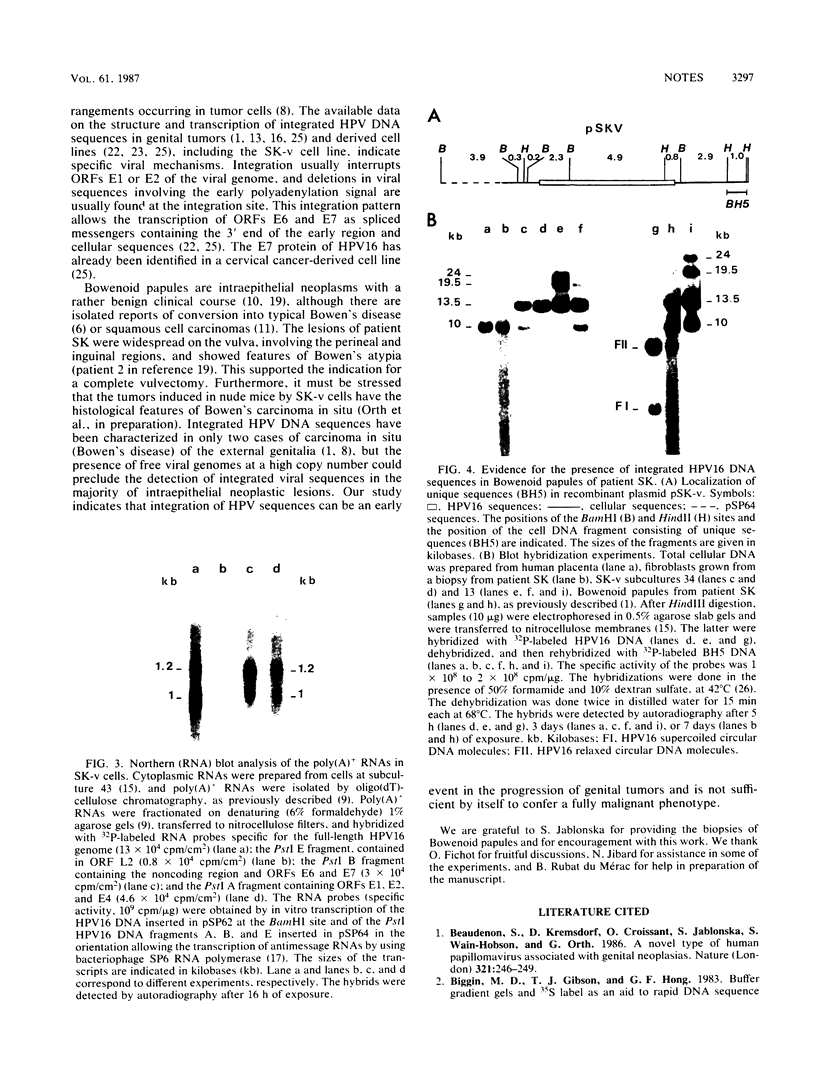
The keratinocyte line SK-v harbors only integrated human papillomavirus type 16 (HPV 16) DNA sequences, although it originated from vulvar Bowenoid papules predominantly containing multiple copies of free HPV 16 genomes. We have cloned a fragment of cell DNA that contains the integrated HPV 16 DNA sequences and have shown that integration interrupts the HPV 16 genome in open reading frames E2 and L2 and creates a deletion of 813 base pairs. This allows the expression of open reading frames E6 and E7, as actually substantiated by Northern (RNA) blot analysis of SK-v RNAs with subgenomic HPV 16 RNA probes. Using a unique flanking cellular DNA sequence as the probe, we have shown that the integration of HPV 16 sequences had already occurred in the premalignant lesions from which the SK-v cell line was derived.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beaudenon S., Kremsdorf D., Croissant O., Jablonska S., Wain-Hobson S., Orth G. A novel type of human papillomavirus associated with genital neoplasias. Nature. 1986 May 15;321(6067):246–249. doi: 10.1038/321246a0. [DOI] [PubMed] [Google Scholar]
- Boshart M., Gissmann L., Ikenberg H., Kleinheinz A., Scheurlen W., zur Hausen H. A new type of papillomavirus DNA, its presence in genital cancer biopsies and in cell lines derived from cervical cancer. EMBO J. 1984 May;3(5):1151–1157. doi: 10.1002/j.1460-2075.1984.tb01944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crum C. P., Mitao M., Levine R. U., Silverstein S. Cervical papillomaviruses segregate within morphologically distinct precancerous lesions. J Virol. 1985 Jun;54(3):675–681. doi: 10.1128/jvi.54.3.675-681.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Villez R. L., Stevens C. S. Bowenoid papules of the genitalia. A case progressing to Bowen's disease. J Am Acad Dermatol. 1980 Aug;3(2):149–152. doi: 10.1016/s0190-9622(80)80252-0. [DOI] [PubMed] [Google Scholar]
- Dürst M., Gissmann L., Ikenberg H., zur Hausen H. A papillomavirus DNA from a cervical carcinoma and its prevalence in cancer biopsy samples from different geographic regions. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3812–3815. doi: 10.1073/pnas.80.12.3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dürst M., Kleinheinz A., Hotz M., Gissmann L. The physical state of human papillomavirus type 16 DNA in benign and malignant genital tumours. J Gen Virol. 1985 Jul;66(Pt 7):1515–1522. doi: 10.1099/0022-1317-66-7-1515. [DOI] [PubMed] [Google Scholar]
- Georges E., Croissant O., Bonneaud N., Orth G. Physical state and transcription of the cottontail rabbit papillomavirus genome in warts and transplantable VX2 and VX7 carcinomas of domestic rabbits. J Virol. 1984 Aug;51(2):530–538. doi: 10.1128/jvi.51.2.530-538.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross G., Hagedorn M., Ikenberg H., Rufli T., Dahlet C., Grosshans E., Gissmann L. Bowenoid papulosis. Presence of human papillomavirus (HPV) structural antigens and of HPV 16-related DNA sequences. Arch Dermatol. 1985 Jul;121(7):858–863. doi: 10.1001/archderm.121.7.858. [DOI] [PubMed] [Google Scholar]
- Ikenberg H., Gissmann L., Gross G., Grussendorf-Conen E. I., zur Hausen H. Human papillomavirus type-16-related DNA in genital Bowen's disease and in Bowenoid papulosis. Int J Cancer. 1983 Nov 15;32(5):563–565. doi: 10.1002/ijc.2910320507. [DOI] [PubMed] [Google Scholar]
- Lehn H., Krieg P., Sauer G. Papillomavirus genomes in human cervical tumors: analysis of their transcriptional activity. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5540–5544. doi: 10.1073/pnas.82.16.5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loenen W. A., Brammar W. J. A bacteriophage lambda vector for cloning large DNA fragments made with several restriction enzymes. Gene. 1980 Aug;10(3):249–259. doi: 10.1016/0378-1119(80)90054-2. [DOI] [PubMed] [Google Scholar]
- Matsukura T., Kanda T., Furuno A., Yoshikawa H., Kawana T., Yoshiike K. Cloning of monomeric human papillomavirus type 16 DNA integrated within cell DNA from a cervical carcinoma. J Virol. 1986 Jun;58(3):979–982. doi: 10.1128/jvi.58.3.979-982.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Obalek S., Jablonska S., Beaudenon S., Walczak L., Orth G. Bowenoid papulosis of the male and female genitalia: risk of cervical neoplasia. J Am Acad Dermatol. 1986 Mar;14(3):433–444. doi: 10.1016/s0190-9622(86)70054-6. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Schneider-Gädicke A., Schwarz E. Different human cervical carcinoma cell lines show similar transcription patterns of human papillomavirus type 18 early genes. EMBO J. 1986 Sep;5(9):2285–2292. doi: 10.1002/j.1460-2075.1986.tb04496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz E., Freese U. K., Gissmann L., Mayer W., Roggenbuck B., Stremlau A., zur Hausen H. Structure and transcription of human papillomavirus sequences in cervical carcinoma cells. Nature. 1985 Mar 7;314(6006):111–114. doi: 10.1038/314111a0. [DOI] [PubMed] [Google Scholar]
- Seedorf K., Krämmer G., Dürst M., Suhai S., Röwekamp W. G. Human papillomavirus type 16 DNA sequence. Virology. 1985 Aug;145(1):181–185. doi: 10.1016/0042-6822(85)90214-4. [DOI] [PubMed] [Google Scholar]
- Smotkin D., Wettstein F. O. Transcription of human papillomavirus type 16 early genes in a cervical cancer and a cancer-derived cell line and identification of the E7 protein. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4680–4684. doi: 10.1073/pnas.83.13.4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee C., Krishnan-Hewlett I., Baker C. C., Schlegel R., Howley P. M. Presence and expression of human papillomavirus sequences in human cervical carcinoma cell lines. Am J Pathol. 1985 Jun;119(3):361–366. [PMC free article] [PubMed] [Google Scholar]