Abstract
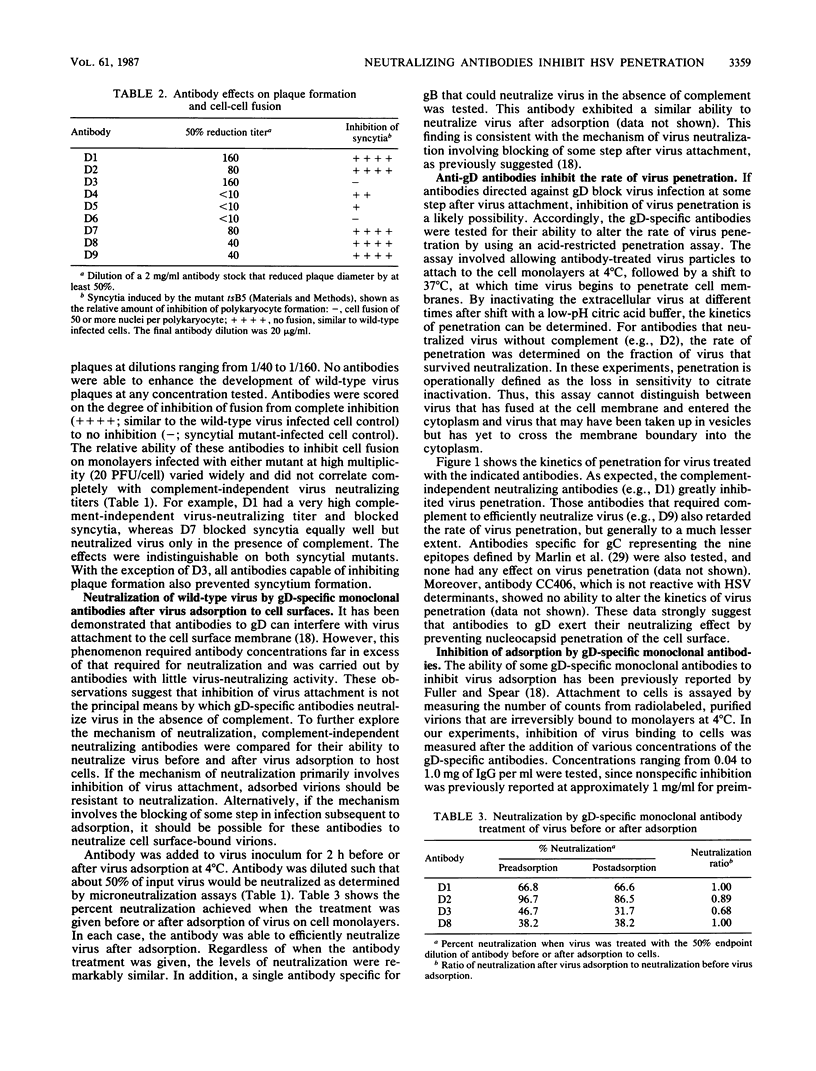
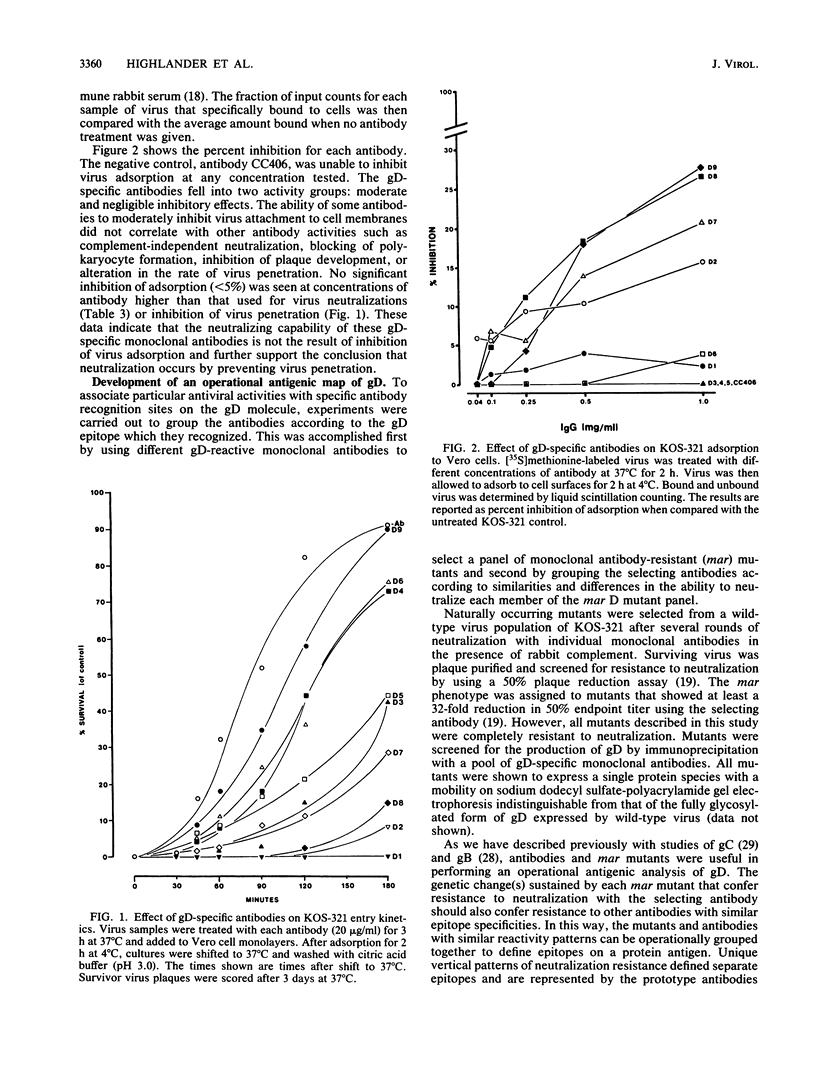
Nine monoclonal antibodies specific for glycoprotein D (gD) of herpes simplex virus type 1 were selected for their ability to neutralize virus in the presence of complement. Four of these antibodies exhibited significant neutralization titers in the absence of complement, suggesting that their epitope specificities are localized to site(s) which contribute to the role of gD in virus infectivity. Each of these antibodies was shown to effectively neutralize virus after virion adsorption to cell surfaces, indicating that neutralization did not involve inhibition of virus attachment. Although some of the monoclonal antibodies partially inhibited adsorption of radiolabeled virions, this effect was only observed at concentrations much higher than that required to neutralize virus and did not correlate with complement-independent virus-neutralizing activity. All of the monoclonal antibodies slowed the rate at which virus entered cells, further suggesting that antibody binding of gD inhibits virus penetration. Experiments were carried out to determine the number of different epitopes recognized by the panel of monoclonal antibodies and to identify epitopes involved in complement-independent virus neutralization. Monoclonal antibody-resistant (mar) mutants were selected by escape from neutralization with individual gD-specific monoclonal antibodies. The reactivity patterns of the mutants and antibodies were then used to construct an operational antigenic map for gD. This analysis identified a minimum of six epitopes on gD that could be grouped into four antigenic sites. Antibodies recognizing four distinct epitopes contained in three antigenic sites were found to neutralize virus in a complement-independent fashion. Moreover, mar mutations in these sites did not affect the processing of gD, rate of virus penetration, or the ability of the virus to replicate at high temperature (39 degrees C). Taken together, these results (i) confirm that gD is a major target antigen for neutralizing antibody, (ii) indicate that the mechanism of neutralization can involve inhibition of virus penetration of the cell surface membrane, and (iii) strongly suggest that gD plays a direct role in the virus entry process.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop G. A., Kümel G., Schwartz S. A., Glorioso J. C. Specificity of human natural killer cells in limiting dilution culture for determinants of herpes simplex virus type 1 glycoproteins. J Virol. 1986 Jan;57(1):294–300. doi: 10.1128/jvi.57.1.294-300.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckmaster E. A., Gompels U., Minson A. Characterisation and physical mapping of an HSV-1 glycoprotein of approximately 115 X 10(3) molecular weight. Virology. 1984 Dec;139(2):408–413. doi: 10.1016/0042-6822(84)90387-8. [DOI] [PubMed] [Google Scholar]
- Bzik D. J., Fox B. A., DeLuca N. A., Person S. Nucleotide sequence of a region of the herpes simplex virus type 1 gB glycoprotein gene: mutations affecting rate of virus entry and cell fusion. Virology. 1984 Aug;137(1):185–190. doi: 10.1016/0042-6822(84)90022-9. [DOI] [PubMed] [Google Scholar]
- Bzik D. J., Fox B. A., DeLuca N. A., Person S. Nucleotide sequence specifying the glycoprotein gene, gB, of herpes simplex virus type 1. Virology. 1984 Mar;133(2):301–314. doi: 10.1016/0042-6822(84)90397-0. [DOI] [PubMed] [Google Scholar]
- Cassai E. N., Sarmiento M., Spear P. G. Comparison of the virion proteins specified by herpes simplex virus types 1 and 2. J Virol. 1975 Nov;16(5):1327–1331. doi: 10.1128/jvi.16.5.1327-1331.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G. H., Isola V. J., Kuhns J., Berman P. W., Eisenberg R. J. Localization of discontinuous epitopes of herpes simplex virus glycoprotein D: use of a nondenaturing ("native" gel) system of polyacrylamide gel electrophoresis coupled with Western blotting. J Virol. 1986 Oct;60(1):157–166. doi: 10.1128/jvi.60.1.157-166.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G. H., Katze M., Hydrean-Stern C., Eisenberg R. J. Type-common CP-1 antigen of herpes simplex virus is associated with a 59,000-molecular-weight envelope glycoprotein. J Virol. 1978 Jul;27(1):172–181. doi: 10.1128/jvi.27.1.172-181.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G. H., Long D., Matthews J. T., May M., Eisenberg R. Glycopeptides of the type-common glycoprotein gD of herpes simplex virus types 1 and 2. J Virol. 1983 Jun;46(3):679–689. doi: 10.1128/jvi.46.3.679-689.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dales S., Silverberg H. Viropexis of herpes simplex virus by HeLa cells. Virology. 1969 Mar;37(3):475–480. doi: 10.1016/0042-6822(69)90232-3. [DOI] [PubMed] [Google Scholar]
- DeLuca N., Bzik D. J., Bond V. C., Person S., Snipes W. Nucleotide sequences of herpes simplex virus type 1 (HSV-1) affecting virus entry, cell fusion, and production of glycoprotein gb (VP7). Virology. 1982 Oct 30;122(2):411–423. doi: 10.1016/0042-6822(82)90240-9. [DOI] [PubMed] [Google Scholar]
- DeLuca N., Bzik D., Person S., Snipes W. Early events in herpes simplex virus type 1 infection: photosensitivity of fluorescein isothiocyanate-treated virions. Proc Natl Acad Sci U S A. 1981 Feb;78(2):912–916. doi: 10.1073/pnas.78.2.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietzschold B., Eisenberg R. J., Ponce de Leon M., Golub E., Hudecz F., Varrichio A., Cohen G. H. Fine structure analysis of type-specific and type-common antigenic sites of herpes simplex virus glycoprotein D. J Virol. 1984 Nov;52(2):431–435. doi: 10.1128/jvi.52.2.431-435.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R. J., Long D., Pereira L., Hampar B., Zweig M., Cohen G. H. Effect of monoclonal antibodies on limited proteolysis of native glycoprotein gD of herpes simplex virus type 1. J Virol. 1982 Feb;41(2):478–488. doi: 10.1128/jvi.41.2.478-488.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R. J., Long D., Ponce de Leon M., Matthews J. T., Spear P. G., Gibson M. G., Lasky L. A., Berman P., Golub E., Cohen G. H. Localization of epitopes of herpes simplex virus type 1 glycoprotein D. J Virol. 1985 Feb;53(2):634–644. doi: 10.1128/jvi.53.2.634-644.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R. J., Ponce de Leon M., Pereira L., Long D., Cohen G. H. Purification of glycoprotein gD of herpes simplex virus types 1 and 2 by use of monoclonal antibody. J Virol. 1982 Mar;41(3):1099–1104. doi: 10.1128/jvi.41.3.1099-1104.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman H. M., Glorioso J. C., Cohen G. H., Hastings J. C., Harris S. L., Eisenberg R. J. Binding of complement component C3b to glycoprotein gC of herpes simplex virus type 1: mapping of gC-binding sites and demonstration of conserved C3b binding in low-passage clinical isolates. J Virol. 1986 Nov;60(2):470–475. doi: 10.1128/jvi.60.2.470-475.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller A. O., Spear P. G. Specificities of monoclonal and polyclonal antibodies that inhibit adsorption of herpes simplex virus to cells and lack of inhibition by potent neutralizing antibodies. J Virol. 1985 Aug;55(2):475–482. doi: 10.1128/jvi.55.2.475-482.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLMES I. H., WATSON D. H. AN ELECTRON MICROSCOPE STUDY OF THE ATTACHMENT AND PENETRATION OF HERPES VIRUS IN BHK21 CELLS. Virology. 1963 Sep;21:112–123. doi: 10.1016/0042-6822(63)90309-x. [DOI] [PubMed] [Google Scholar]
- HUANG A. S., WAGNER R. R. PENETRATION OF HERPES SIMPLEX VIRUS INTO HUMAN EPIDERMOID CELLS. Proc Soc Exp Biol Med. 1964 Aug-Sep;116:863–869. doi: 10.3181/00379727-116-29392. [DOI] [PubMed] [Google Scholar]
- Holland T. C., Person S. Ammonium chloride inhibits cell fusion induced by syn mutants of herpes simplex virus type 1. J Virol. 1977 Jul;23(1):213–215. doi: 10.1128/jvi.23.1.213-215.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess R. W., Watson D. H. Herpes simplex virus-specific polypeptides studied by polyacrylamide gel electrophoresis of immune precipitates. J Gen Virol. 1974 Feb;22(2):171–185. doi: 10.1099/0022-1317-22-2-171. [DOI] [PubMed] [Google Scholar]
- Inada T., Mims C. A. Mouse Ia antigens are receptors for lactate dehydrogenase virus. Nature. 1984 May 3;309(5963):59–61. doi: 10.1038/309059a0. [DOI] [PubMed] [Google Scholar]
- Johnson D. C., Wittels M., Spear P. G. Binding to cells of virosomes containing herpes simplex virus type 1 glycoproteins and evidence for fusion. J Virol. 1984 Oct;52(1):238–247. doi: 10.1128/jvi.52.1.238-247.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little S. P., Jofre J. T., Courtney R. J., Schaffer P. A. A virion-associated glycoprotein essential for infectivity of herpes simplex virus type 1. Virology. 1981 Nov;115(1):149–160. doi: 10.1016/0042-6822(81)90097-0. [DOI] [PubMed] [Google Scholar]
- Mapoles J. E., Krah D. L., Crowell R. L. Purification of a HeLa cell receptor protein for group B coxsackieviruses. J Virol. 1985 Sep;55(3):560–566. doi: 10.1128/jvi.55.3.560-566.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlin S. D., Highlander S. L., Holland T. C., Levine M., Glorioso J. C. Antigenic variation (mar mutations) in herpes simplex virus glycoprotein B can induce temperature-dependent alterations in gB processing and virus production. J Virol. 1986 Jul;59(1):142–153. doi: 10.1128/jvi.59.1.142-153.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlin S. D., Holland T. C., Levine M., Glorioso J. C. Epitopes of herpes simplex virus type 1 glycoprotein gC are clustered in two distinct antigenic sites. J Virol. 1985 Jan;53(1):128–136. doi: 10.1128/jvi.53.1.128-136.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews J. T., Cohen G. H., Eisenberg R. J. Synthesis and processing of glycoprotein D of herpes simplex virus types 1 and 2 in an in vitro system. J Virol. 1983 Nov;48(2):521–533. doi: 10.1128/jvi.48.2.521-533.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeoch D. J., Dolan A., Donald S., Rixon F. J. Sequence determination and genetic content of the short unique region in the genome of herpes simplex virus type 1. J Mol Biol. 1985 Jan 5;181(1):1–13. doi: 10.1016/0022-2836(85)90320-1. [DOI] [PubMed] [Google Scholar]
- Minson A. C., Hodgman T. C., Digard P., Hancock D. C., Bell S. E., Buckmaster E. A. An analysis of the biological properties of monoclonal antibodies against glycoprotein D of herpes simplex virus and identification of amino acid substitutions that confer resistance to neutralization. J Gen Virol. 1986 Jun;67(Pt 6):1001–1013. doi: 10.1099/0022-1317-67-6-1001. [DOI] [PubMed] [Google Scholar]
- Morgan C., Rose H. M., Mednis B. Electron microscopy of herpes simplex virus. I. Entry. J Virol. 1968 May;2(5):507–516. doi: 10.1128/jvi.2.5.507-516.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble A. G., Lee G. T., Sprague R., Parish M. L., Spear P. G. Anti-gD monoclonal antibodies inhibit cell fusion induced by herpes simplex virus type 1. Virology. 1983 Aug;129(1):218–224. doi: 10.1016/0042-6822(83)90409-9. [DOI] [PubMed] [Google Scholar]
- Noseworthy J. H., Fields B. N., Dichter M. A., Sobotka C., Pizer E., Perry L. L., Nepom J. T., Greene M. I. Cell receptors for the mammalian reovirus. I. Syngeneic monoclonal anti-idiotypic antibody identifies a cell surface receptor for reovirus. J Immunol. 1983 Nov;131(5):2533–2538. [PubMed] [Google Scholar]
- Para M. F., Baucke R. B., Spear P. G. Immunoglobulin G(Fc)-binding receptors on virions of herpes simplex virus type 1 and transfer of these receptors to the cell surface by infection. J Virol. 1980 May;34(2):512–520. doi: 10.1128/jvi.34.2.512-520.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Para M. F., Parish M. L., Noble A. G., Spear P. G. Potent neutralizing activity associated with anti-glycoprotein D specificity among monoclonal antibodies selected for binding to herpes simplex virions. J Virol. 1985 Aug;55(2):483–488. doi: 10.1128/jvi.55.2.483-488.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawls W. E., Balachandran N., Sisson G., Watson R. J. Localization of a type-specific antigenic site on herpes simplex virus type 2 glycoprotein D. J Virol. 1984 Jul;51(1):263–265. doi: 10.1128/jvi.51.1.263-265.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandri-Goldin R. M., Levine M., Glorioso J. C. Method for induction of mutations in physically defined regions of the herpes simplex virus genome. J Virol. 1981 Apr;38(1):41–49. doi: 10.1128/jvi.38.1.41-49.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarmiento M., Haffey M., Spear P. G. Membrane proteins specified by herpes simplex viruses. III. Role of glycoprotein VP7(B2) in virion infectivity. J Virol. 1979 Mar;29(3):1149–1158. doi: 10.1128/jvi.29.3.1149-1158.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Showalter S. D., Zweig M., Hampar B. Monoclonal antibodies to herpes simplex virus type 1 proteins, including the immediate-early protein ICP 4. Infect Immun. 1981 Dec;34(3):684–692. doi: 10.1128/iai.34.3.684-692.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim C., Watson D. H. The role of type specific and cross reacting structural antigens in the neutralization of herpes simplex virus types 1 and 2. J Gen Virol. 1973 May;19(2):217–233. doi: 10.1099/0022-1317-19-2-217. [DOI] [PubMed] [Google Scholar]
- Spear P. G. Membrane proteins specified by herpes simplex viruses. I. Identification of four glycoprotein precursors and their products in type 1-infected cells. J Virol. 1976 Mar;17(3):991–1008. doi: 10.1128/jvi.17.3.991-1008.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear P. G., Roizman B. Proteins specified by herpes simplex virus. V. Purification and structural proteins of the herpesvirion. J Virol. 1972 Jan;9(1):143–159. doi: 10.1128/jvi.9.1.143-159.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahlne A., Svennerholm B., Lycke E. Evidence for herpes simplex virus type-selective receptors on cellular plasma membranes. J Gen Virol. 1979 Jul;44(1):217–225. doi: 10.1099/0022-1317-44-1-217. [DOI] [PubMed] [Google Scholar]
- Vahlne A., Svennerholm B., Sandberg M., Hamberger A., Lycke E. Differences in attachment between herpes simplex type 1 and type 2 viruses to neurons and glial cells. Infect Immun. 1980 Jun;28(3):675–680. doi: 10.1128/iai.28.3.675-680.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson R. J. DNA sequence of the Herpes simplex virus type 2 glycoprotein D gene. Gene. 1983 Dec;26(2-3):307–312. doi: 10.1016/0378-1119(83)90203-2. [DOI] [PubMed] [Google Scholar]
- Watson R. J., Weis J. H., Salstrom J. S., Enquist L. W. Herpes simplex virus type-1 glycoprotein D gene: nucleotide sequence and expression in Escherichia coli. Science. 1982 Oct 22;218(4570):381–384. doi: 10.1126/science.6289440. [DOI] [PubMed] [Google Scholar]
- Weber P. C., Levine M., Glorioso J. C. Rapid identification of nonessential genes of herpes simplex virus type 1 by Tn5 mutagenesis. Science. 1987 May 1;236(4801):576–579. doi: 10.1126/science.3033824. [DOI] [PubMed] [Google Scholar]


