Abstract
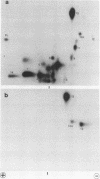
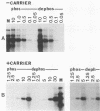
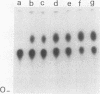
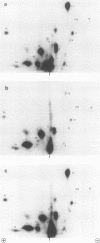
The effect of phosphorylation on the ability of simian virus 40 large T antigen to stimulate DNA synthesis in vitro was tested. Treatment of affinity-purified large T antigen with calf intestinal alkaline phosphatase resulted in the removal of 70 to 80% of the phosphate residues. Only serine-bound phosphate residues were affected. Phosphatase-treated large T antigen stimulated in vitro DNA synthesis fourfold over the untreated control. The stimulation was strongest at early times of DNA replication. At later times, DNA replication proceeded at equal rates with dephosphorylated and untreated large T antigen. The ATPase activity of large T antigen was not affected by phosphatase treatment. The origin-binding activity of large T antigen was tested over a wide range of large T antigen to DNA ratios, including DNA excess, and in the presence and absence of carrier DNA. Under no condition was an effect of dephosphorylation of large T antigen on its DNA-binding activity observed. These findings might indicate that phosphorylation at serine residues modulates the interaction of large T antigen with cellular factors. During DNA synthesis large T antigen was substantially rephosphorylated by kinases in the HeLa cell extract. As shown by two-dimensional peptide mapping, this phosphorylation occurred at all known in vivo sites. No phosphatase and protease activities were detectable in the HeLa cell extract.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumann E. A. DNA-binding properties of phosphorylated and dephosphorylated D2-T antigen, a simian-virus-40 T-antigen-related protein. Eur J Biochem. 1985 Mar 15;147(3):495–501. doi: 10.1111/j.0014-2956.1985.00495.x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Dixon R. A., Nathans D. Purification of simian virus 40 large T antigen by immunoaffinity chromatography. J Virol. 1985 Mar;53(3):1001–1004. doi: 10.1128/jvi.53.3.1001-1004.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow E., Crawford L. V., Pim D. C., Williamson N. M. Monoclonal antibodies specific for simian virus 40 tumor antigens. J Virol. 1981 Sep;39(3):861–869. doi: 10.1128/jvi.39.3.861-869.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalderon D., Smith A. E. In vitro mutagenesis of a putative DNA binding domain of SV40 large-T. Virology. 1984 Nov;139(1):109–137. doi: 10.1016/0042-6822(84)90334-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Li J. J., Kelly T. J. Simian virus 40 DNA replication in vitro. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6973–6977. doi: 10.1073/pnas.81.22.6973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. J., Kelly T. J. Simian virus 40 DNA replication in vitro: specificity of initiation and evidence for bidirectional replication. Mol Cell Biol. 1985 Jun;5(6):1238–1246. doi: 10.1128/mcb.5.6.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. J., Peden K. W., Dixon R. A., Kelly T. Functional organization of the simian virus 40 origin of DNA replication. Mol Cell Biol. 1986 Apr;6(4):1117–1128. doi: 10.1128/mcb.6.4.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay R. D. Binding of a simian virus 40 T antigen-related protein to DNA. J Mol Biol. 1981 Jan 25;145(3):471–488. doi: 10.1016/0022-2836(81)90540-4. [DOI] [PubMed] [Google Scholar]
- Mohr I. J., Stillman B., Gluzman Y. Regulation of SV40 DNA replication by phosphorylation of T antigen. EMBO J. 1987 Jan;6(1):153–160. doi: 10.1002/j.1460-2075.1987.tb04733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paucha E., Kalderon D., Harvey R. W., Smith A. E. Simian virus 40 origin DNA-binding domain on large T antigen. J Virol. 1986 Jan;57(1):50–64. doi: 10.1128/jvi.57.1.50-64.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidtmann K. H., Echle B., Walter G. Simian virus 40 large T antigen is phosphorylated at multiple sites clustered in two separate regions. J Virol. 1982 Oct;44(1):116–133. doi: 10.1128/jvi.44.1.116-133.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidtmann K. H., Hardung M., Echle B., Walter G. DNA-binding activity of simian virus 40 large T antigen correlates with a distinct phosphorylation state. J Virol. 1984 Apr;50(1):1–12. doi: 10.1128/jvi.50.1.1-12.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidtmann K. H. Phosphorylation of simian virus 40 large T antigen: cytoplasmic and nuclear phophorylation sites differ in their metabolic stability. Virology. 1986 Apr 15;150(1):85–95. [PubMed] [Google Scholar]
- Scheidtmann K. H., Schickedanz J., Walter G., Lanford R. E., Butel J. S. Differential phosphorylation of cytoplasmic and nuclear variants of simian virus 40 large T antigen encoded by simian virus 40-adenovirus 7 hybrid viruses. J Virol. 1984 May;50(2):636–640. doi: 10.1128/jvi.50.2.636-640.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schickedanz J., Scheidtmann K. H., Walter G. Kinetics of nuclear transport and oligomerization of simian virus 40 large T antigen. Virology. 1986 Jan 15;148(1):47–57. doi: 10.1016/0042-6822(86)90402-2. [DOI] [PubMed] [Google Scholar]
- Schneider C., Newman R. A., Sutherland D. R., Asser U., Greaves M. F. A one-step purification of membrane proteins using a high efficiency immunomatrix. J Biol Chem. 1982 Sep 25;257(18):10766–10769. [PubMed] [Google Scholar]
- Shaw S. B., Tegtmeyer P. Binding of dephosphorylated A protein to SV40 DNA. Virology. 1981 Nov;115(1):88–96. doi: 10.1016/0042-6822(81)90091-x. [DOI] [PubMed] [Google Scholar]
- Simanis V., Lane D. P. An immunoaffinity purification procedure for SV40 large T antigen. Virology. 1985 Jul 15;144(1):88–100. doi: 10.1016/0042-6822(85)90308-3. [DOI] [PubMed] [Google Scholar]
- Simmons D. T., Chou W., Rodgers K. Phosphorylation downregulates the DNA-binding activity of simian virus 40 T antigen. J Virol. 1986 Dec;60(3):888–894. doi: 10.1128/jvi.60.3.888-894.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons D. T. DNA-binding region of the simian virus 40 tumor antigen. J Virol. 1986 Mar;57(3):776–785. doi: 10.1128/jvi.57.3.776-785.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons D. T. Stepwise phosphorylation of the NH2-terminal region of the simian virus 40 large T antigen. J Biol Chem. 1984 Jul 10;259(13):8633–8640. [PubMed] [Google Scholar]
- Smale S. T., Tjian R. T-antigen-DNA polymerase alpha complex implicated in simian virus 40 DNA replication. Mol Cell Biol. 1986 Nov;6(11):4077–4087. doi: 10.1128/mcb.6.11.4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl H., Dröge P., Knippers R. DNA helicase activity of SV40 large tumor antigen. EMBO J. 1986 Aug;5(8):1939–1944. doi: 10.1002/j.1460-2075.1986.tb04447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillman B. W., Gluzman Y. Replication and supercoiling of simian virus 40 DNA in cell extracts from human cells. Mol Cell Biol. 1985 Aug;5(8):2051–2060. doi: 10.1128/mcb.5.8.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillman B., Gerard R. D., Guggenheimer R. A., Gluzman Y. T antigen and template requirements for SV40 DNA replication in vitro. EMBO J. 1985 Nov;4(11):2933–2939. doi: 10.1002/j.1460-2075.1985.tb04026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjian R., Robbins A., Clark R. Catalytic properties of the SV40 large T antigen. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):103–111. doi: 10.1101/sqb.1980.044.01.012. [DOI] [PubMed] [Google Scholar]
- Tjian R., Robbins A. Enzymatic activities associated with a purified simian virus 40 T antigen-related protein. Proc Natl Acad Sci U S A. 1979 Feb;76(2):610–614. doi: 10.1073/pnas.76.2.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Roy F., Fransen L., Fiers W. Metabolic turnover of phosphorylation sites in simian virus 40 large T antigen. J Virol. 1983 Jan;45(1):442–446. doi: 10.1128/jvi.45.1.442-446.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wobbe C. R., Dean F., Weissbach L., Hurwitz J. In vitro replication of duplex circular DNA containing the simian virus 40 DNA origin site. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5710–5714. doi: 10.1073/pnas.82.17.5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wold M. S., Li J. J., Kelly T. J. Initiation of simian virus 40 DNA replication in vitro: large-tumor-antigen- and origin-dependent unwinding of the template. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3643–3647. doi: 10.1073/pnas.84.11.3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Roy F., Fransen L., Fiers W. Improved localization of phosphorylation sites in simian virus 40 large T antigen. J Virol. 1983 Jan;45(1):315–331. doi: 10.1128/jvi.45.1.315-331.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]