Abstract
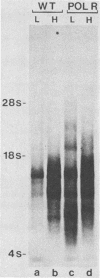
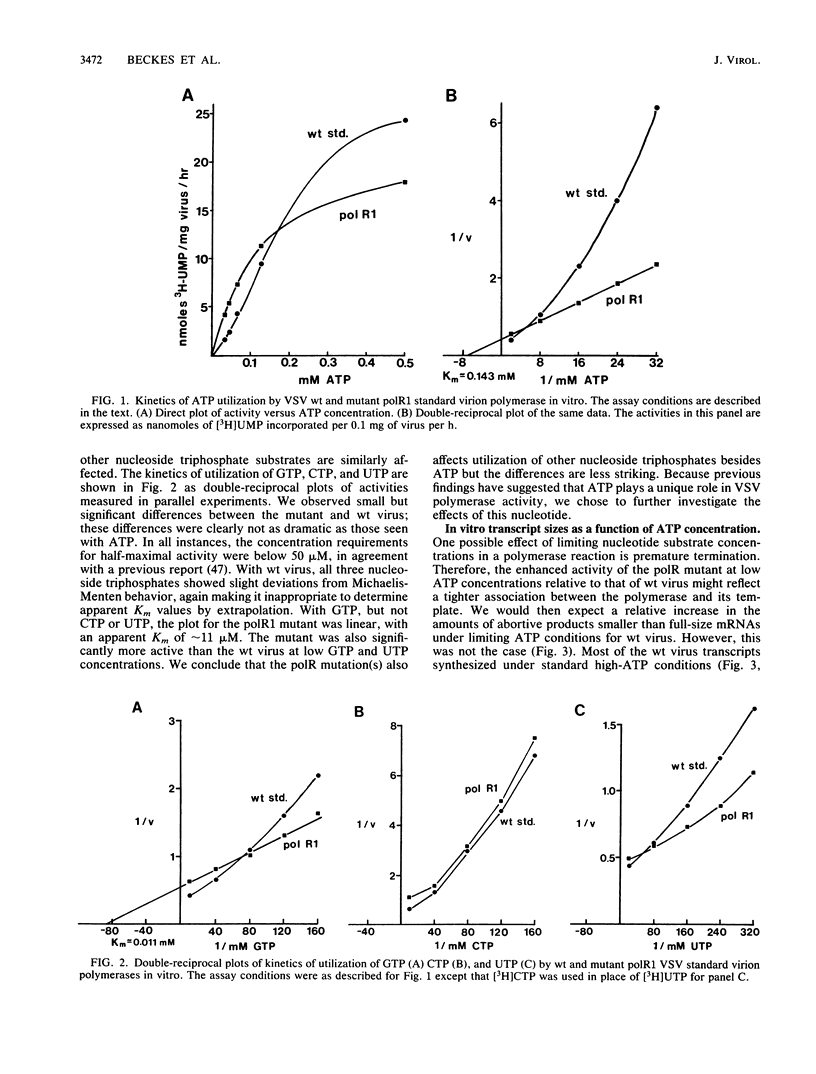
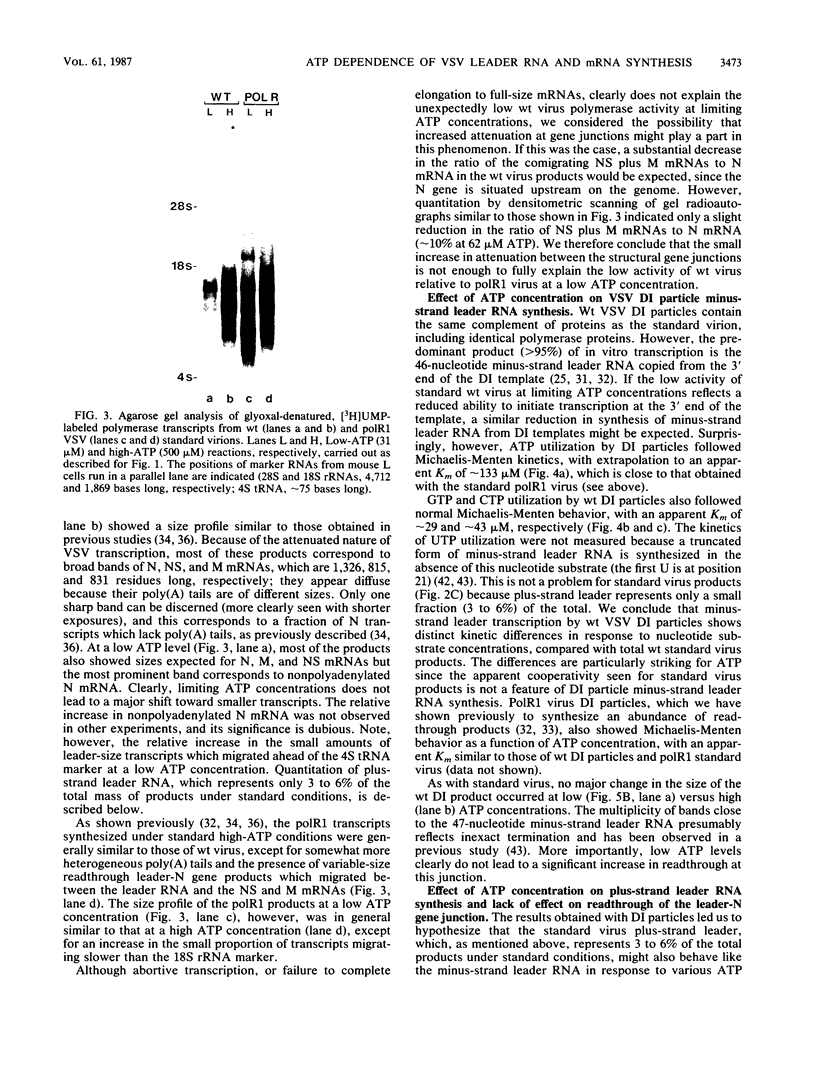
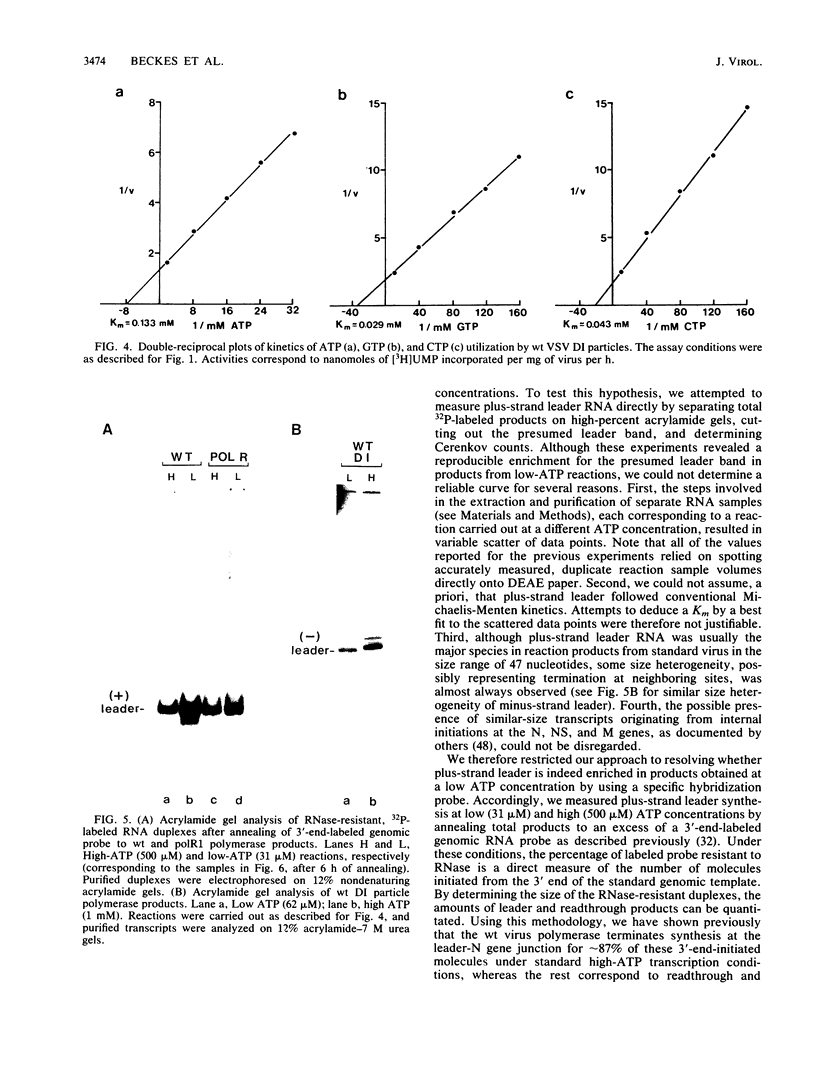
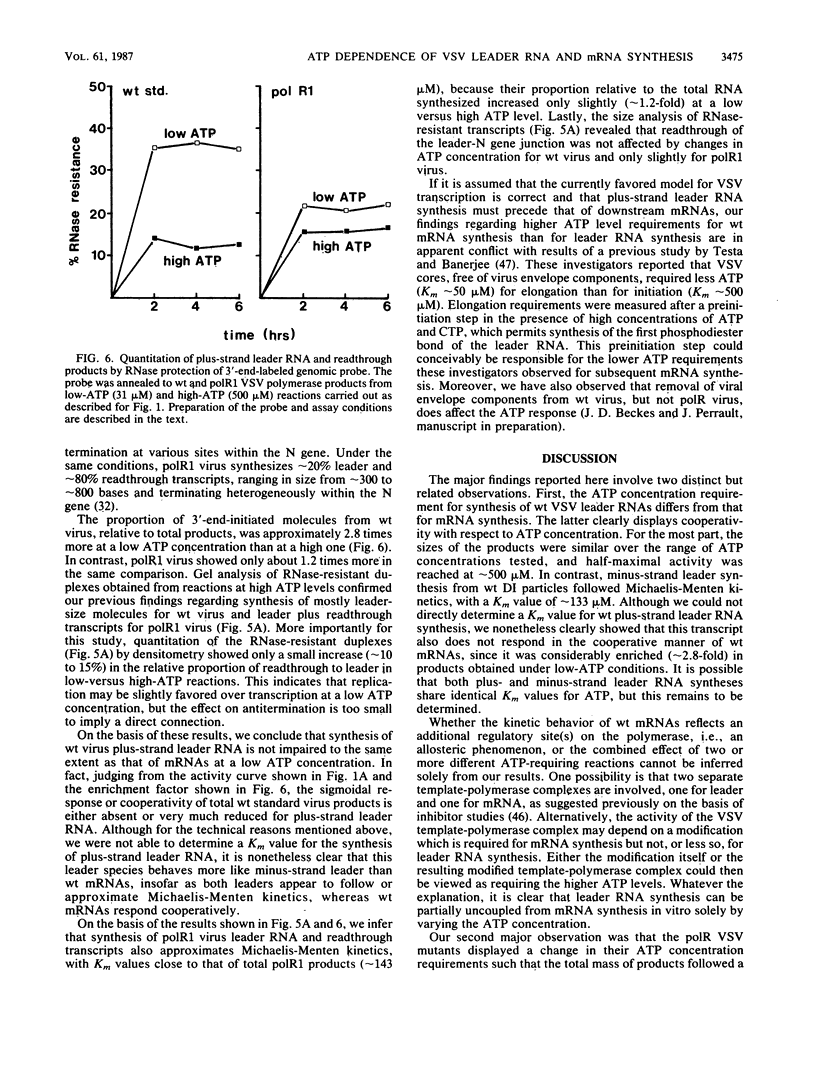
Cleavage of the beta-gamma bond of ATP is required for wild-type (wt) vesicular stomatitis virus transcription in vitro. Recent findings have established that a domain-specific phosphorylation of the virus NS protein is necessary for activity. We report here that RNA synthesis catalyzed by purified standard wt virions responded cooperatively to various ATP concentrations, with half-maximal activity at approximately 500 microM. In contrast, mutant polR1 standard virions and wt defective interfering particles both showed conventional Michaelis-Menten kinetic profiles with Km values of approximately 143 and approximately 133 microM, respectively. The former synthesize readthrough products of the leader-N gene junction in addition to plus-strand leader RNA and mRNAs, whereas the latter synthesize only minus-strand leader RNA. The cooperative response of wt virus products, however, was specific to mRNAs; the small fraction of the total products corresponding to plus-strand leader approximated Michaelis-Menten behavior. Since the unique phenotype of the polR mutants correlates with the synthesis of replicationlike products in vitro, the affected ATP-requiring function most likely regulates both transcription and replication. We suggest that this mutated function involves phosphorylation of viral proteins.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnheiter H., Davis N. L., Wertz G., Schubert M., Lazzarini R. A. Role of the nucleocapsid protein in regulating vesicular stomatitis virus RNA synthesis. Cell. 1985 May;41(1):259–267. doi: 10.1016/0092-8674(85)90079-0. [DOI] [PubMed] [Google Scholar]
- Banerjee A. K. The transcription complex of vesicular stomatitis virus. Cell. 1987 Feb 13;48(3):363–364. doi: 10.1016/0092-8674(87)90184-x. [DOI] [PubMed] [Google Scholar]
- Banerjee A. K. Transcription and replication of rhabdoviruses. Microbiol Rev. 1987 Mar;51(1):66–87. doi: 10.1128/mr.51.1.66-87.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. H., Obijeski J. F., Simpson R. W. Transcription of the influenza ribonucleic acid genome by a virion polymerase. I. Optimal conditions for in vitro activity of the ribonucleic acid-dependent ribonucleic acid polymerase. J Virol. 1971 Jul;8(1):66–73. doi: 10.1128/jvi.8.1.66-73.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg B. M., Leppert M., Kolakofsky D. Interaction of VSV leader RNA and nucleocapsid protein may control VSV genome replication. Cell. 1981 Mar;23(3):837–845. doi: 10.1016/0092-8674(81)90448-7. [DOI] [PubMed] [Google Scholar]
- Both G. W., Moyer S. A., Banerjee A. K. Translation and identification of the viral mRNA species isolated from subcellular fractions of vesicular stomatitis virus-infected cells. J Virol. 1975 Apr;15(4):1012–1019. doi: 10.1128/jvi.15.4.1012-1019.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunick D., Zandomeni R., Ackerman S., Weinmann R. Mechanism of RNA polymerase II--specific initiation of transcription in vitro: ATP requirement and uncapped runoff transcripts. Cell. 1982 Jul;29(3):877–886. doi: 10.1016/0092-8674(82)90449-4. [DOI] [PubMed] [Google Scholar]
- Chanda P. K., Banerjee A. K. Inhibition of vesicular stomatitis virus transcriptase in vitro by phosphonoformate and ara-ATP. Virology. 1980 Dec;107(2):562–566. doi: 10.1016/0042-6822(80)90327-x. [DOI] [PubMed] [Google Scholar]
- Chanda P. K., Roy J., Banerjee A. K. In vitro synthesis of genome length complementary RNA of vesicular stomatitis virus in the presence of inosine 5'-triphosphate. Virology. 1983 Aug;129(1):225–229. doi: 10.1016/0042-6822(83)90410-5. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay D., Banerjee A. K. Phosphorylation within a specific domain of the phosphoprotein of vesicular stomatitis virus regulates transcription in vitro. Cell. 1987 May 8;49(3):407–414. doi: 10.1016/0092-8674(87)90293-5. [DOI] [PubMed] [Google Scholar]
- Chinchar V. G., Amesse L. S., Portner A. Linked transcripts of the genes for leader and N message are synthesized in vitro by vesicular stomatitis virus. Biochem Biophys Res Commun. 1982 Apr 29;105(4):1296–1302. doi: 10.1016/0006-291x(82)90927-5. [DOI] [PubMed] [Google Scholar]
- Clinton G. M., Guerina N. G., Guo H. Y., Huang A. S. Host-dependent phosphorylation and kinase activity associated with vesicular stomatitis virus. J Biol Chem. 1982 Mar 25;257(6):3313–3319. [PubMed] [Google Scholar]
- Colonno R. J., Banerjee A. K. Mapping and initiation studies on the leader RNA of vesicular stomatitis virus. Virology. 1977 Mar;77(1):260–268. doi: 10.1016/0042-6822(77)90423-8. [DOI] [PubMed] [Google Scholar]
- De B. P., Banerjee A. K. Requirements and functions of vesicular stomatitis virus L and NS proteins in the transcription process in vitro. Biochem Biophys Res Commun. 1985 Jan 16;126(1):40–49. doi: 10.1016/0006-291x(85)90568-6. [DOI] [PubMed] [Google Scholar]
- Dmitrieva T. M., Eremeeva T. P., Alatortseva G. I., Agol V. I. On the mechanism of single-stranded RNA synthesis by encephalomyocarditis virus replication complexes: preferential inhibition by adenylyl (beta, gamma-methylene)-diphosphonate. FEBS Lett. 1980 Jun 16;115(1):19–22. doi: 10.1016/0014-5793(80)80717-4. [DOI] [PubMed] [Google Scholar]
- Emerson S. U. Reconstitution studies detect a single polymerase entry site on the vesicular stomatitis virus genome. Cell. 1982 Dec;31(3 Pt 2):635–642. doi: 10.1016/0092-8674(82)90319-1. [DOI] [PubMed] [Google Scholar]
- Emerson S. U., Yu Y. Both NS and L proteins are required for in vitro RNA synthesis by vesicular stomatitis virus. J Virol. 1975 Jun;15(6):1348–1356. doi: 10.1128/jvi.15.6.1348-1356.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershowitz A., Boone R. F., Moss B. Multiple roles for ATP in the synthesis and processing of mRNA by vaccinia virus: specific inhibitory effects of adenosine (beta,gamma-imido) triphosphate. J Virol. 1978 Aug;27(2):399–408. doi: 10.1128/jvi.27.2.399-408.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill D. S., Chattopadhyay D., Banerjee A. K. Identification of a domain within the phosphoprotein of vesicular stomatitis virus that is essential for transcription in vitro. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8873–8877. doi: 10.1073/pnas.83.23.8873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green T. L., Emerson S. U. Effect of the beta-gamma phosphate bond of ATP on synthesis of leader RNA and mRNAs of vesicular stomatitis virus. J Virol. 1984 Apr;50(1):255–257. doi: 10.1128/jvi.50.1.255-257.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon S. A., Marnell L. L., Summers D. F. The major ribonucleoprotein-associated protein kinase of vesicular stomatitis virus is a host cell protein. J Biol Chem. 1983 Dec 25;258(24):15283–15290. [PubMed] [Google Scholar]
- Herman R. C., Lazzarini R. A. Vesicular stomatitis virus RNA polymerase can read through the boundary between the leader and N genes in vitro. J Virol. 1981 May;38(2):792–796. doi: 10.1128/jvi.38.2.792-796.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imblum R. L., Wagner R. R. Protein kinase and phosphoproteins of vesicular stomatitis virus. J Virol. 1974 Jan;13(1):113–124. doi: 10.1128/jvi.13.1.113-124.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iverson L. E., Rose J. K. Localized attenuation and discontinuous synthesis during vesicular stomatitis virus transcription. Cell. 1981 Feb;23(2):477–484. doi: 10.1016/0092-8674(81)90143-4. [DOI] [PubMed] [Google Scholar]
- Lazzarini R. A., Keene J. D., Schubert M. The origins of defective interfering particles of the negative-strand RNA viruses. Cell. 1981 Oct;26(2 Pt 2):145–154. doi: 10.1016/0092-8674(81)90298-1. [DOI] [PubMed] [Google Scholar]
- Leppert M., Rittenhouse L., Perrault J., Summers D. F., Kolakofsky D. Plus and minus strand leader RNAs in negative strand virus-infected cells. Cell. 1979 Nov;18(3):735–747. doi: 10.1016/0092-8674(79)90127-2. [DOI] [PubMed] [Google Scholar]
- Masters P. S., Banerjee A. K. Phosphoprotein NS of vesicular stomatitis virus: phosphorylated states and transcriptional activities of intracellular and virion forms. Virology. 1986 Oct 30;154(2):259–270. doi: 10.1016/0042-6822(86)90452-6. [DOI] [PubMed] [Google Scholar]
- Morrow C. D., Hocko J., Navab M., Dasgupta A. ATP is required for initiation of poliovirus RNA synthesis in vitro: demonstration of tyrosine-phosphate linkage between in vitro-synthesized RNA and genome-linked protein. J Virol. 1984 May;50(2):515–523. doi: 10.1128/jvi.50.2.515-523.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer S. A., Summers D. F. Phosphorylation of vesicular stomatitis virus in vivo and in vitro. J Virol. 1974 Feb;13(2):455–465. doi: 10.1128/jvi.13.2.455-465.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton J. T., Davis N. L., Wertz G. W. N protein alone satisfies the requirement for protein synthesis during RNA replication of vesicular stomatitis virus. J Virol. 1984 Feb;49(2):303–309. doi: 10.1128/jvi.49.2.303-309.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrault J., Clinton G. M., McClure M. A. RNP template of vesicular stomatitis virus regulates transcription and replication functions. Cell. 1983 Nov;35(1):175–185. doi: 10.1016/0092-8674(83)90220-9. [DOI] [PubMed] [Google Scholar]
- Perrault J., Leavitt R. W. Characterization of snap-back RNAs in vesicular stomatitis defective interfering virus particles. J Gen Virol. 1978 Jan;38(1):21–34. doi: 10.1099/0022-1317-38-1-21. [DOI] [PubMed] [Google Scholar]
- Perrault J., McLear P. W. ATP dependence of vesicular stomatitis virus transcription initiation and modulation by mutation in the nucleocapsid protein. J Virol. 1984 Sep;51(3):635–642. doi: 10.1128/jvi.51.3.635-642.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrault J. Origin and replication of defective interfering particles. Curr Top Microbiol Immunol. 1981;93:151–207. doi: 10.1007/978-3-642-68123-3_7. [DOI] [PubMed] [Google Scholar]
- Perrault J., Semler B. L. Internal genome deletions in two distinct classes of defective interfering particles of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6191–6195. doi: 10.1073/pnas.76.12.6191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purchio A. F., Wells S. K., Collett M. S. Increase in the phosphotransferase specific activity of purified Rous sarcoma virus pp60v-src protein after incubation with ATP plus Mg2+. Mol Cell Biol. 1983 Sep;3(9):1589–1597. doi: 10.1128/mcb.3.9.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy P., Bishop D. H. Nucleoside triphosphate phosphotransferase. A new enzyme activity of oncogenic and non-oncogenic "budding" viruses. Biochim Biophys Acta. 1971 Apr 14;235(1):191–206. doi: 10.1016/0005-2744(71)90047-7. [DOI] [PubMed] [Google Scholar]
- Schubert M., Keene J. D., Lazzarini R. A., Emerson S. U. The complete sequence of a unique RNA species synthesized by a DI particle of VSV. Cell. 1978 Sep;15(1):103–112. doi: 10.1016/0092-8674(78)90086-7. [DOI] [PubMed] [Google Scholar]
- Semler B. L., Perrault J., Abelson J., Holland J. J. Sequence of a RNA templated by the 3'-OH RNA terminus of defective interfering particles of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4704–4708. doi: 10.1073/pnas.75.10.4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinacore M. S., Lucas-Lenard J. The effect of the vesicular stomatitis virus-associated protein kinase on viral mRNA transcription in vitro. Virology. 1982 Sep;121(2):404–413. doi: 10.1016/0042-6822(82)90178-7. [DOI] [PubMed] [Google Scholar]
- Soria M., Little S. P., Huang A. S. Characterization of vesicular stomatitis virus nucleocapsids. I. Complementary 40 S RNA molecules in nucleocapsids. Virology. 1974 Sep;61(1):270–280. doi: 10.1016/0042-6822(74)90261-x. [DOI] [PubMed] [Google Scholar]
- Sánchez A., De B. P., Banerjee A. K. In vitro phosphorylation of NS protein by the L protein of vesicular stomatitis virus. J Gen Virol. 1985 May;66(Pt 5):1025–1036. doi: 10.1099/0022-1317-66-5-1025. [DOI] [PubMed] [Google Scholar]
- Talib S., Hearst J. E. Initiation of RNA synthesis in vitro by vesicular stomatitis virus: single internal initiation in the presence of aurintricarboxylic acid and vanadyl ribonucleoside complexes. Nucleic Acids Res. 1983 Oct 25;11(20):7031–7042. doi: 10.1093/nar/11.20.7031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa D., Banerjee A. K. Initiation of RNA synthesis in vitro by vesicular stomatitis virus. Role of ATP. J Biol Chem. 1979 Mar 25;254(6):2053–2058. [PubMed] [Google Scholar]
- Testa D., Chanda P. K., Banerjee A. K. In vitro synthesis of the full-length complement of the negative-strand genome RNA of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1980 Jan;77(1):294–298. doi: 10.1073/pnas.77.1.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa D., Chanda P. K., Banerjee A. K. Unique mode of transcription in vitro by Vesicular stomatitis virus. Cell. 1980 Aug;21(1):267–275. doi: 10.1016/0092-8674(80)90134-8. [DOI] [PubMed] [Google Scholar]
- Thomas D., Newcomb W. W., Brown J. C., Wall J. S., Hainfeld J. F., Trus B. L., Steven A. C. Mass and molecular composition of vesicular stomatitis virus: a scanning transmission electron microscopy analysis. J Virol. 1985 May;54(2):598–607. doi: 10.1128/jvi.54.2.598-607.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]