Abstract
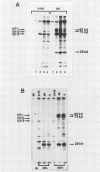
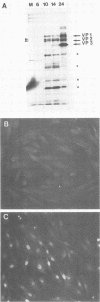
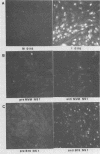
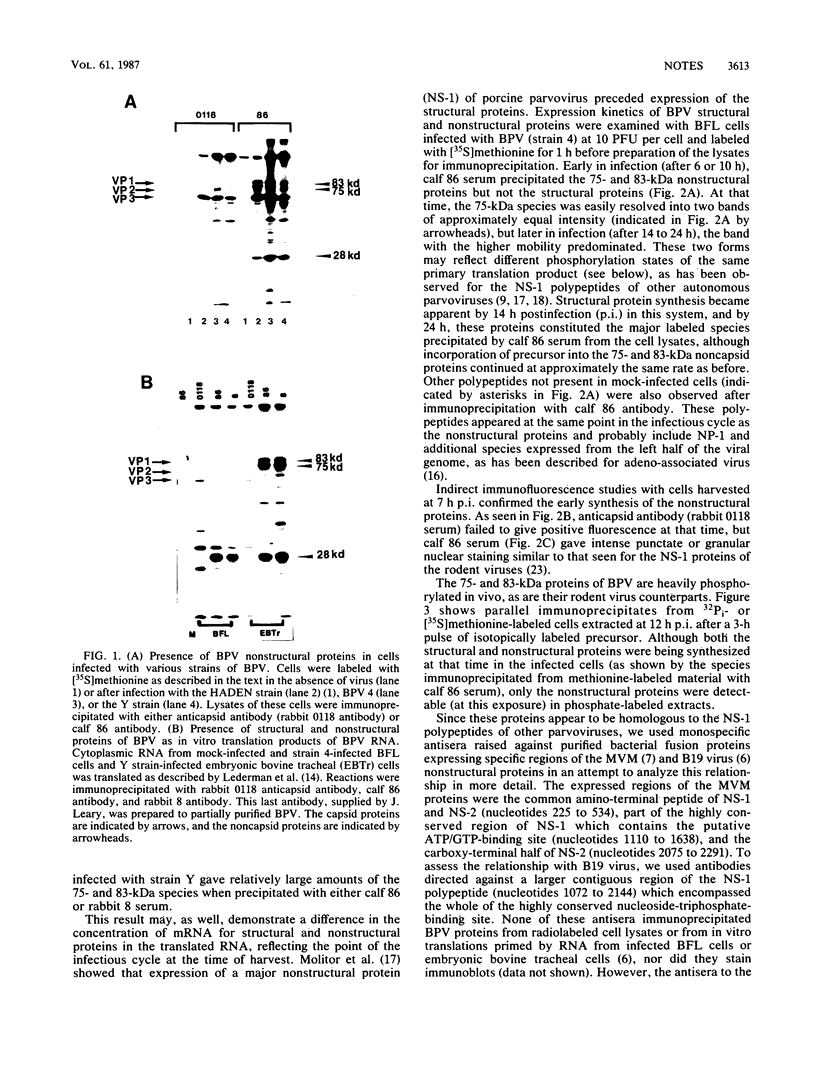
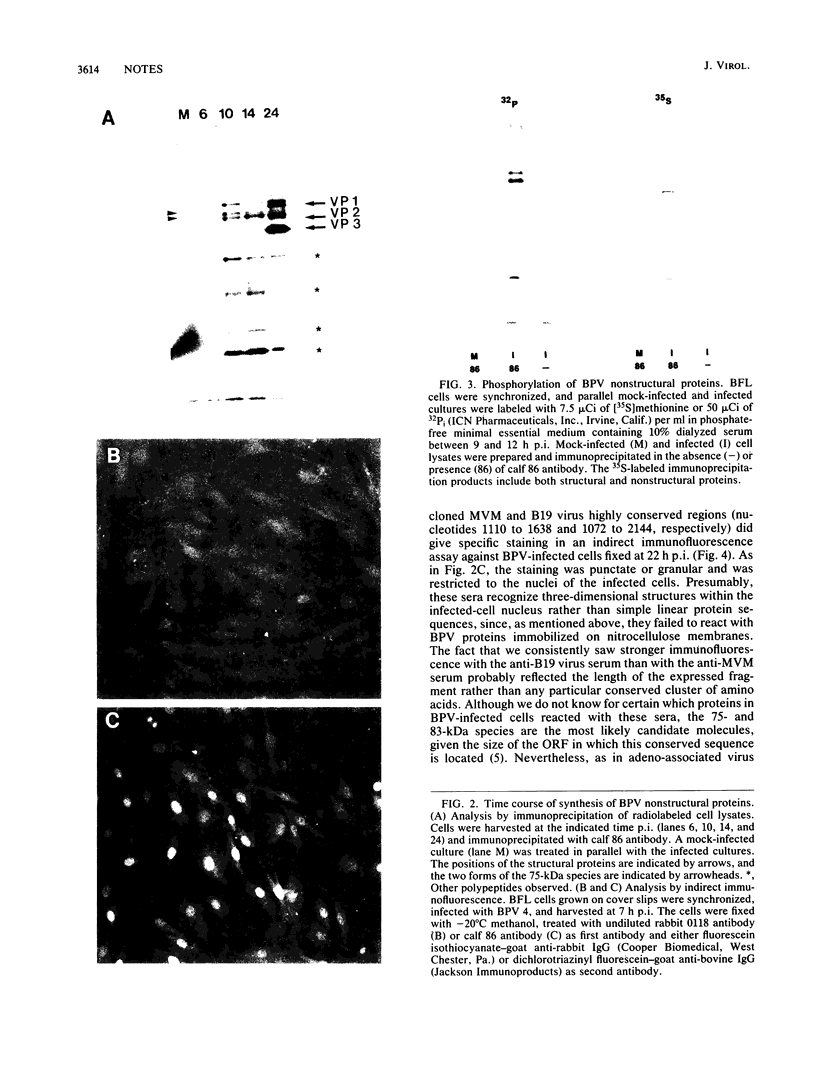
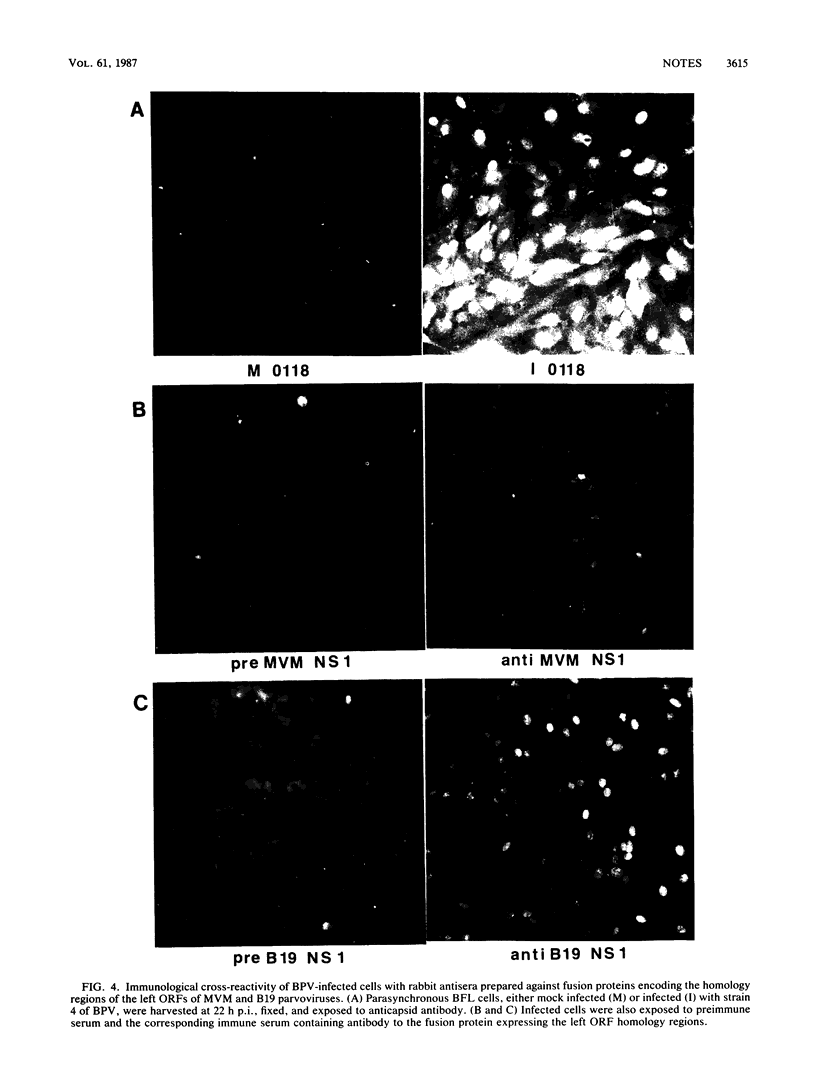
Two nonstructural proteins of bovine parvovirus (BPV) with apparent molecular sizes of 75,000 and 83,000 daltons have been detected. The proteins were immunoprecipitated from lung cells infected with various isolates of BPV and from in vitro translations of infected cell mRNA. These proteins were expressed as nuclear phosphoproteins and were synthesized early in infection, before the peak of capsid protein synthesis. Early in infection, the 75-kilodalton-size species could be resolved into two bands of equal intensity, but later in infection, the lower-molecular-size form predominated. Antibodies directed against bacterial fusion proteins encoding amino acid sequences from a highly conserved region of the NS-1 polypeptides of two other parvoviruses, minute virus of mice and the human virus B19, gave specific nuclear fluorescence with BPV-infected cells, although the antibodies failed to immunoprecipitate any viral proteins. The noncapsid proteins appear to be homologous to the previously characterized NS-1 proteins of other autonomous parvoviruses.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABINANTI F. R., WARFIELD M. S. Recovery of a hemadsorbing virus (HADEN) from the gastrointestinal tract of calves. Virology. 1961 Jun;14:288–289. doi: 10.1016/0042-6822(61)90206-9. [DOI] [PubMed] [Google Scholar]
- Bates R. C., Storz J., Reed D. E. Isolation and comparison of bovine parvoviruses. J Infect Dis. 1972 Nov;126(5):531–536. doi: 10.1093/infdis/126.5.531. [DOI] [PubMed] [Google Scholar]
- Bloom M. E., Race R. E., Wolfinbarger J. B. Identification of a nonvirion protein of Aleutian disease virus: mink with Aleutian disease have antibody to both virion and nonvirion proteins. J Virol. 1982 Aug;43(2):608–616. doi: 10.1128/jvi.43.2.608-616.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buller R. M., Rose J. A. Characterization of adenovirus-associated virus-induced polypeptides in KB cells. J Virol. 1978 Jan;25(1):331–338. doi: 10.1128/jvi.25.1.331-338.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K. C., Shull B. C., Moses E. A., Lederman M., Stout E. R., Bates R. C. Complete nucleotide sequence and genome organization of bovine parvovirus. J Virol. 1986 Dec;60(3):1085–1097. doi: 10.1128/jvi.60.3.1085-1097.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotmore S. F., McKie V. C., Anderson L. J., Astell C. R., Tattersall P. Identification of the major structural and nonstructural proteins encoded by human parvovirus B19 and mapping of their genes by procaryotic expression of isolated genomic fragments. J Virol. 1986 Nov;60(2):548–557. doi: 10.1128/jvi.60.2.548-557.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotmore S. F., Sturzenbecker L. J., Tattersall P. The autonomous parvovirus MVM encodes two nonstructural proteins in addition to its capsid polypeptides. Virology. 1983 Sep;129(2):333–343. doi: 10.1016/0042-6822(83)90172-1. [DOI] [PubMed] [Google Scholar]
- Cotmore S. F., Tattersall P. Organization of nonstructural genes of the autonomous parvovirus minute virus of mice. J Virol. 1986 Jun;58(3):724–732. doi: 10.1128/jvi.58.3.724-732.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotmore S. F., Tattersall P. The NS-1 polypeptide of the autonomous parvovirus MVM is a nuclear phosphoprotein. Virus Res. 1986 May;4(3):243–250. doi: 10.1016/0168-1702(86)90003-1. [DOI] [PubMed] [Google Scholar]
- Cotmore S. F., Tattersall P. The autonomously replicating parvoviruses of vertebrates. Adv Virus Res. 1987;33:91–174. doi: 10.1016/s0065-3527(08)60317-6. [DOI] [PubMed] [Google Scholar]
- Hermonat P. L., Labow M. A., Wright R., Berns K. I., Muzyczka N. Genetics of adeno-associated virus: isolation and preliminary characterization of adeno-associated virus type 2 mutants. J Virol. 1984 Aug;51(2):329–339. doi: 10.1128/jvi.51.2.329-339.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lederman M., Bates R. C., Stout E. R. In vitro and in vivo studies of bovine parvovirus proteins. J Virol. 1983 Oct;48(1):10–17. doi: 10.1128/jvi.48.1.10-17.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederman M., Patton J. T., Stout E. R., Bates R. C. Virally coded noncapsid protein associated with bovine parvovirus infection. J Virol. 1984 Feb;49(2):315–318. doi: 10.1128/jvi.49.2.315-318.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunaga Y., Matsuno S. Structural and nonstructural proteins of a rabbit parvovirus. J Virol. 1983 Feb;45(2):627–633. doi: 10.1128/jvi.45.2.627-633.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson E., Trempe J. P., Carter B. J. Identification of the trans-acting Rep proteins of adeno-associated virus by antibodies to a synthetic oligopeptide. J Virol. 1986 Dec;60(3):823–832. doi: 10.1128/jvi.60.3.823-832.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molitor T. W., Joo H. S., Collett M. S. Identification and characterization of a porcine parvovirus nonstructural polypeptide. J Virol. 1985 Sep;55(3):554–559. doi: 10.1128/jvi.55.3.554-559.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradiso P. R. Identification of multiple forms of the noncapsid parvovirus protein NCVP1 in H-1 parvovirus-infected cells. J Virol. 1984 Oct;52(1):82–87. doi: 10.1128/jvi.52.1.82-87.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parris D. S., Bates R. C. Effect of bovine parvovirus replication on DNA, RNA, and protein synthesis in S phase cells. Virology. 1976 Aug;73(1):72–78. doi: 10.1016/0042-6822(76)90061-1. [DOI] [PubMed] [Google Scholar]
- Rhode S. L., 3rd, Paradiso P. R. Parvovirus genome: nucleotide sequence of H-1 and mapping of its genes by hybrid-arrested translation. J Virol. 1983 Jan;45(1):173–184. doi: 10.1128/jvi.45.1.173-184.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode S. L., 3rd trans-Activation of parvovirus P38 promoter by the 76K noncapsid protein. J Virol. 1985 Sep;55(3):886–889. doi: 10.1128/jvi.55.3.886-889.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shade R. O., Blundell M. C., Cotmore S. F., Tattersall P., Astell C. R. Nucleotide sequence and genome organization of human parvovirus B19 isolated from the serum of a child during aplastic crisis. J Virol. 1986 Jun;58(3):921–936. doi: 10.1128/jvi.58.3.921-936.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tratschin J. D., Miller I. L., Carter B. J. Genetic analysis of adeno-associated virus: properties of deletion mutants constructed in vitro and evidence for an adeno-associated virus replication function. J Virol. 1984 Sep;51(3):611–619. doi: 10.1128/jvi.51.3.611-619.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]