Abstract
The bacteriophage T4 rII genes and the lambda rex (r exclusion) genes interact; rII mutants are unable to productively infect rex+ lambda lysogens. The relationship between rex and rII has been found to be quantitative, and plasmid clones of rex have excluded not only rII mutants but T4 wild type and most other bacteriophages as well. Mutations in the T4 motA gene substantially reversed exclusion of T4 by rex.
Full text
PDF
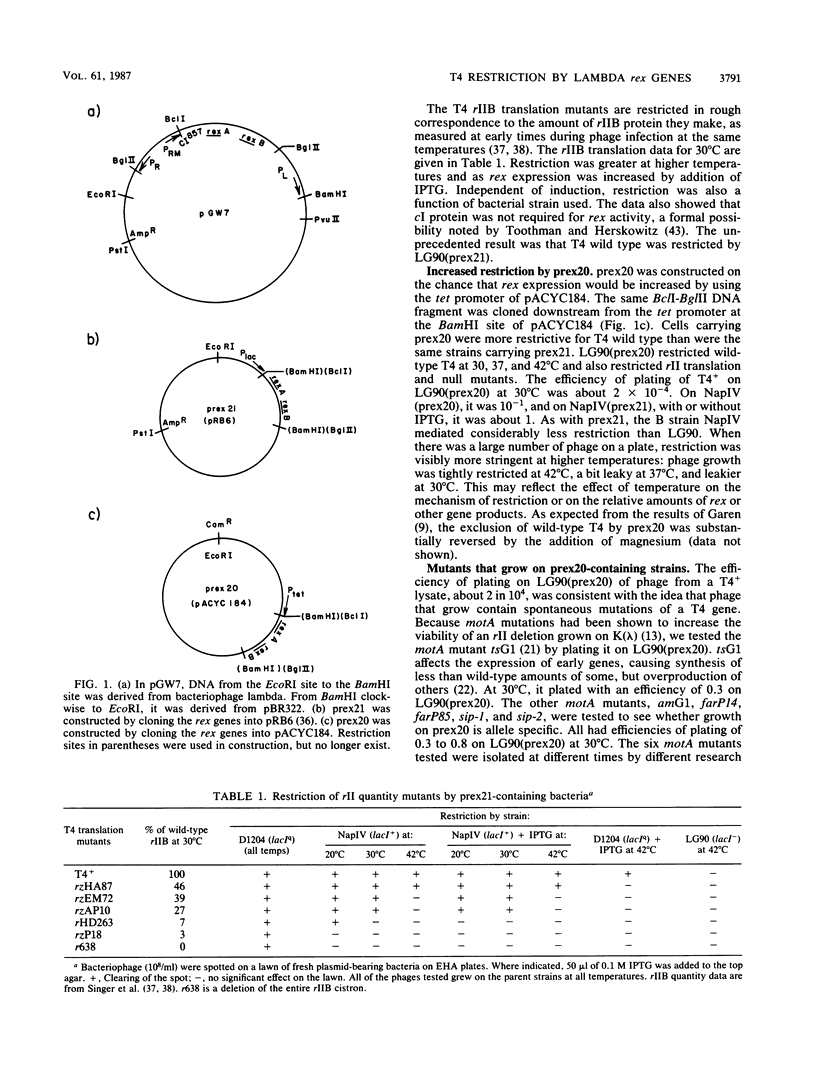
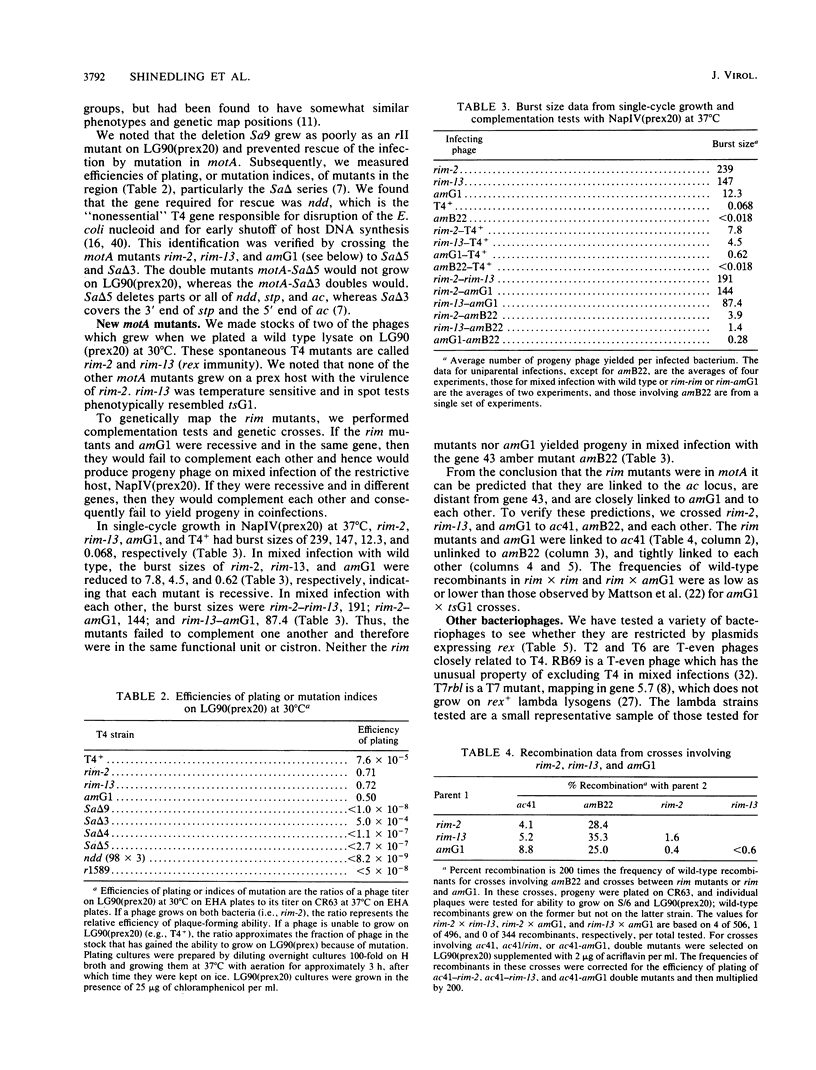
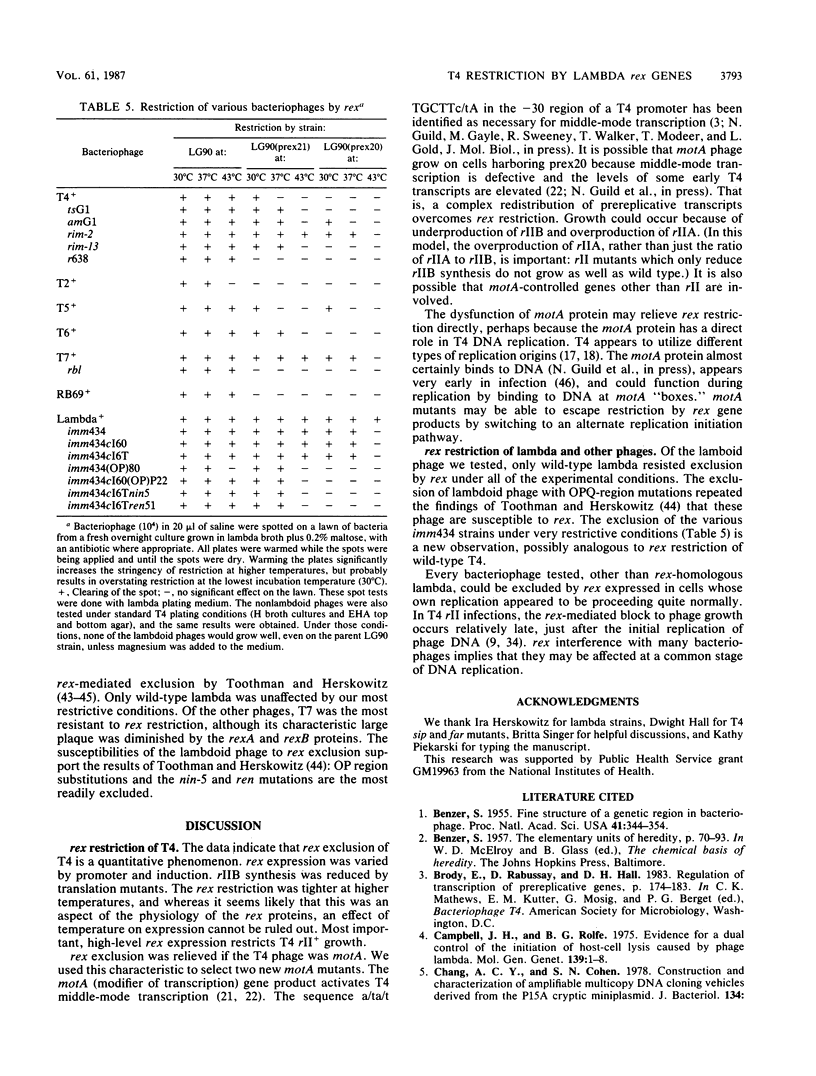

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benzer S. FINE STRUCTURE OF A GENETIC REGION IN BACTERIOPHAGE. Proc Natl Acad Sci U S A. 1955 Jun 15;41(6):344–354. doi: 10.1073/pnas.41.6.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRICK F. H., BARNETT L., BRENNER S., WATTS-TOBIN R. J. General nature of the genetic code for proteins. Nature. 1961 Dec 30;192:1227–1232. doi: 10.1038/1921227a0. [DOI] [PubMed] [Google Scholar]
- Campbell J. H., Rolfe B. G. Evidence for a dual control of the initiation of host-cell lysis caused by phage lambda. Mol Gen Genet. 1975 Aug 5;139(1):1–8. doi: 10.1007/BF00267990. [DOI] [PubMed] [Google Scholar]
- Depew R. E., Snopek T. J., Cozzarelli N. R. Characterization of a new class of deletions of the D region of the bacteriophage T4 genome. Virology. 1975 Mar;64(1):144–145. doi: 10.1016/0042-6822(75)90086-0. [DOI] [PubMed] [Google Scholar]
- Dunn J. J., Studier F. W. Complete nucleotide sequence of bacteriophage T7 DNA and the locations of T7 genetic elements. J Mol Biol. 1983 Jun 5;166(4):477–535. doi: 10.1016/s0022-2836(83)80282-4. [DOI] [PubMed] [Google Scholar]
- GAREN A. Physiological effects of rII mutations in bacteriophage T4. Virology. 1961 Jun;14:151–163. doi: 10.1016/0042-6822(61)90190-8. [DOI] [PubMed] [Google Scholar]
- Guarente L., Lauer G., Roberts T. M., Ptashne M. Improved methods for maximizing expression of a cloned gene: a bacterium that synthesizes rabbit beta-globin. Cell. 1980 Jun;20(2):543–553. doi: 10.1016/0092-8674(80)90640-6. [DOI] [PubMed] [Google Scholar]
- Hall D. H., Snyder R. D. Suppressors of mutations in the rII gene of bacteriophage T4 affect promoter utilization. Genetics. 1981 Jan;97(1):1–9. doi: 10.1093/genetics/97.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershey A. D. Mutation of Bacteriophage with Respect to Type of Plaque. Genetics. 1946 Nov;31(6):620–640. doi: 10.1093/genetics/31.6.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homyk T., Jr, Rodriguez A., Weil J. Characterization of T4 mutants that partially suppress the inability of T4rII to grow in Lambda lysogens. Genetics. 1976 Jul;83(3 PT2):477–487. [PMC free article] [PubMed] [Google Scholar]
- Homyk T., Jr, Weil J. Deletion analysis of two nonessential regions of the T4 genome. Virology. 1974 Oct;61(2):505–523. doi: 10.1016/0042-6822(74)90286-4. [DOI] [PubMed] [Google Scholar]
- Jacquemin-Sablon A., Lanni Y. T. Lambda-repressed mutants of bacteriophage T5. I. Isolation and genetical characterization. Virology. 1973 Nov;56(1):230–237. doi: 10.1016/0042-6822(73)90302-4. [DOI] [PubMed] [Google Scholar]
- Koerner J. F., Snustad D. P. Shutoff of host macromolecular synthesis after T-even bacteriophage infection. Microbiol Rev. 1979 Jun;43(2):199–223. doi: 10.1128/mr.43.2.199-223.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuzer K. N., Alberts B. M. A defective phage system reveals bacteriophage T4 replication origins that coincide with recombination hot spots. Proc Natl Acad Sci U S A. 1985 May;82(10):3345–3349. doi: 10.1073/pnas.82.10.3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuzer K. N., Alberts B. M. Characterization of a defective phage system for the analysis of bacteriophage T4 DNA replication origins. J Mol Biol. 1986 Mar 20;188(2):185–198. doi: 10.1016/0022-2836(86)90303-7. [DOI] [PubMed] [Google Scholar]
- Landsmann J., Kröger M., Hobom G. The rex region of bacteriophage lambda: two genes under three-way control. Gene. 1982 Nov;20(1):11–24. doi: 10.1016/0378-1119(82)90083-x. [DOI] [PubMed] [Google Scholar]
- Lauer G., Pastrana R., Sherley J., Ptashne M. Construction of overproducers of the bacteriophage 434 repressor and cro proteins. J Mol Appl Genet. 1981;1(2):139–147. [PubMed] [Google Scholar]
- Mattson T., Richardson J., Goodin D. Mutant of bacteriophage T4D affecting expression of many early genes. Nature. 1974 Jul 5;250(461):48–50. doi: 10.1038/250048a0. [DOI] [PubMed] [Google Scholar]
- Mattson T., Van Houwe G., Epstein R. H. Isolation and characterization of conditional lethal mutations in the mot gene of bacteriophage T4. J Mol Biol. 1978 Dec 15;126(3):551–570. doi: 10.1016/0022-2836(78)90058-x. [DOI] [PubMed] [Google Scholar]
- Matz K., Schmandt M., Gussin G. N. The rex gene of bacteriophage lambda is really two genes. Genetics. 1982 Nov;102(3):319–327. doi: 10.1093/genetics/102.3.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson M. A., Singer B. S., Gold L., Pribnow D. Mutations that detoxify an aberrant T4 membrane protein. J Mol Biol. 1981 Jul 5;149(3):377–403. doi: 10.1016/0022-2836(81)90478-2. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. Z., Gold L. M. Bacteriophage T4 gene expression. Evidence for two classes of prereplicative cistrons. J Biol Chem. 1973 Aug 10;248(15):5502–5511. [PubMed] [Google Scholar]
- Pao C. C., Speyer J. F. Mutants of T7 bacteriophage inhibited by lambda prophage. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3642–3646. doi: 10.1073/pnas.72.9.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirrotta V., Ineichen K., Walz A. An unusual RNA polymerase binding site in the immunity region of phage lambda. Mol Gen Genet. 1980;180(2):369–376. doi: 10.1007/BF00425850. [DOI] [PubMed] [Google Scholar]
- Pribnow D., Sigurdson D. C., Gold L., Singer B. S., Napoli C., Brosius J., Dull T. J., Noller H. F. rII cistrons of bacteriophage T4. DNA sequence around the intercistronic divide and positions of genetic landmarks. J Mol Biol. 1981 Jul 5;149(3):337–376. doi: 10.1016/0022-2836(81)90477-0. [DOI] [PubMed] [Google Scholar]
- Ptashne M., Backman K., Humayun M. Z., Jeffrey A., Maurer R., Meyer B., Sauer R. T. Autoregulation and function of a repressor in bacteriophage lambda. Science. 1976 Oct 8;194(4261):156–161. doi: 10.1126/science.959843. [DOI] [PubMed] [Google Scholar]
- Pulitzer J. F., Coppo A., Caruso M. Host--virus interactions in the control of T4 prereplicative transcription. II. Interaction between tabC (rho) mutants and T4 mot mutants. J Mol Biol. 1979 Dec 25;135(4):979–997. doi: 10.1016/0022-2836(79)90523-0. [DOI] [PubMed] [Google Scholar]
- Russell R. L., Huskey R. J. Partial exclusion between T-even bacteriophages: an incipient genetic isolation mechanism. Genetics. 1974 Dec;78(4):989–1014. doi: 10.1093/genetics/78.4.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEINBERG C. M., EDGAR R. S. A critical test of a current theory of genetic recombination in bacteriophage. Genetics. 1962 Feb;47:187–208. doi: 10.1093/genetics/47.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadler J. R., Tecklenburg M., Betz J. L. Plasmids containing many tandem copies of a synthetic lactose operator. Gene. 1980 Feb;8(3):279–300. doi: 10.1016/0378-1119(80)90005-0. [DOI] [PubMed] [Google Scholar]
- Sauerbier W., Puck S. M., Bräutigam A. R., Hirsch-Kauffmann M. Control of gene function in bacteriophage T4. I. Ribonucleic acid and deoxyribonucleic acid metabolism in T4rII-infected lambda-lysogenic hosts. J Virol. 1969 Nov;4(5):742–752. doi: 10.1128/jvi.4.5.742-752.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinedling S., Singer B. S., Gayle M., Pribnow D., Jarvis E., Edgar B., Gold L. Sequences and studies of bacteriophage T4 rII mutants. J Mol Biol. 1987 Jun 5;195(3):471–480. doi: 10.1016/0022-2836(87)90176-8. [DOI] [PubMed] [Google Scholar]
- Shinedling S., Walker L. T., Gold L. Cloning the complete rIIB gene of bacteriophage T4 and some observations concerning its middle promoters. J Virol. 1986 Nov;60(2):787–792. doi: 10.1128/jvi.60.2.787-792.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer B. S., Gold L. A mutation that confers temperature sensitivity on the translation of rIIB in bacteriophage T4. J Mol Biol. 1976 May 25;103(3):627–646. doi: 10.1016/0022-2836(76)90221-7. [DOI] [PubMed] [Google Scholar]
- Singer B. S., Gold L., Shinedling S. T., Colkitt M., Hunter L. R., Pribnow D., Nelson M. A. Analysis in vivo of translational mutants of the rIIB cistron of bacteriophage T4. J Mol Biol. 1981 Jul 5;149(3):405–432. doi: 10.1016/0022-2836(81)90479-4. [DOI] [PubMed] [Google Scholar]
- Snustad D. P., Bursch C. J., Parson K. A., Hefeneider S. H. Mutants of bacteriophage T4 deficient in the ability to induce nuclear disruption: shutoff of host DNA and protein synthesis gene dosage experiments, identification of a restrictive host, and possible biological significance. J Virol. 1976 Apr;18(1):268–288. doi: 10.1128/jvi.18.1.268-288.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takacs B. J., Rosenbusch J. P. Modification of Escherichia coli membranes in the prereplicative phase of phage T4 infection. Specificity of association and quantitation of bound phage proteins. J Biol Chem. 1975 Mar 25;250(6):2339–2350. [PubMed] [Google Scholar]
- Toothman P., Herskowitz I. Rex-dependent exclusion of lambdoid phages. I. Prophage requirements for exclusion. Virology. 1980 Apr 15;102(1):133–146. doi: 10.1016/0042-6822(80)90076-8. [DOI] [PubMed] [Google Scholar]
- Toothman P., Herskowitz I. Rex-dependent exclusion of lambdoid phages. II. Determinants of sensitivity to exclusion. Virology. 1980 Apr 15;102(1):147–160. doi: 10.1016/0042-6822(80)90077-x. [DOI] [PubMed] [Google Scholar]
- Toothman P., Herskowitz I. Rex-dependent exclusion of lambdoid phages. III. Physiology of the abortive infection. Virology. 1980 Apr 15;102(1):161–171. doi: 10.1016/0042-6822(80)90078-1. [DOI] [PubMed] [Google Scholar]
- Uzan M., Leautey J., d'Aubenton-Carafa Y., Brody E. Identification and biosynthesis of the bacteriophage T4 mot regulatory protein. EMBO J. 1983;2(7):1207–1212. doi: 10.1002/j.1460-2075.1983.tb01568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


