Metal-free chiral Brønsted acid catalysis has been a growing area of research for the past decade. Several chiral Brønsted acids such as urea/thiourea and TADDOL have been reported as an activator of electrophiles via hydrogen bonding.1,2 In 2004, the research groups of Akiyama3 and Terada4 independently reported a different type of activation of electrophiles by way of protonation with moderately strong phosphoric acids derived from chiral BINOLs. Following these seminal studies, it soon became clear that chiral phosphoric acids possessed tremendous potential for application in the development of novel asymmetric processes.5 However, due to the relatively low acidity of phosphoric acids, their utility has been limited to more basic nitrogen-based electrophiles such as imines or aziridines. The activation of aldehydes and ketones by chiral phosphoric acids has been very rare. Our group reported the first activation of carbonyl compounds with chiral phosphoric acids by introducing the N-trifluoromethane-sulfonyl (NTf) group into phosphoric acid.6,7 Continuing efforts to make the utility of chiral phosphoric acid catalysts more general in organic synthesis require the design of a new chiral Brønsted acid with higher acidity. In general, acidity increases as it descends in a column of the periodic table due to better stabilization of the conjugate base in a larger size atom. For example, the pKa values of PhOH, PhSH, and PhSeH in DMSO are 18.0, 10.3, and 7.1, respectively.8 With this in mind, we expected that substitution of the oxygen in the P=O bond in an N-triflyl phosphoramide with sulfur or selenium would increase the reactivity of the Brønsted acid.9
Enantioselective protonation of prochiral enol derivatives is an attractive route for the preparation of optically active α-carbonyl compounds.10 However, it is difficult to control the enantioselectivity in an acidic condition because of the bonding flexibility between the proton and its chiral counterion and the orientational flexibility of the proton. Our group overcame these difficulties with the Lewis acid assisted chiral Brønsted acid (LBA) system.11,12 Although the LBA provided an excellent solution for asymmetric protonation reactions of silyl enol ethers, to the best of our knowledge, metal-free chiral Brønsted acid catalyzed enantioselective protonations of silyl enol ethers have never been reported.13 This is probably due to the unavailability of chiral Brønsted acids with suitable acidity. Herein we describe the preparation of a new designer chiral Brønsted acid and its application to the enantioselective protonation reaction of silyl enol ethers.
Chiral N-triflyl thio- or selenophosphoramides 4a–e and 5 were synthesized from the optically active BINOL derivatives by thio- or selenophosphorylation with PSCl3 or PCl3 followed by oxidation with selenium powder and amidation of the resultant thio- or selenophosphoryl chlorides by NH2Tf.14 X-ray crystallography revealed, as suggested in the molecular structure of 4c (Table 1), 4c has a P=S double bond rather than a P=N double bond, which implies the proton is located on the nitrogen atom, instead of the sulfur atom bonded to the phosphorus atom.14
Table 1.
Reactivity for the Protonation Reactions
 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| entry | catalyst | time (h) | % yielda | erb | entry | catalyst | time (h) | % yielda | erb |
| 1 | 1 | 96 | NR | ND | 3 | 3 | 4.5 | >99 (98) | 77:23 (S) |
| 4 | 4c | 3.5 | >99 (97) | 89:11 (S) | |||||
| 2 | 2 | 96 | trace | ND | 5 | 5 | 3.5 | >99 (97) | 86:14 (S) |
Yield was measured by 1H NMR analysis, and the isolated yields are shown in parentheses.
Enantiomeric ratio (er) was determined by HPLC analysis. NR and ND mean no reaction and not determined, respectively.
To compare the catalytic reactivity of various chiral Brønsted acids 1–5, the enantioselective protonations of the silyl enol ether 6a were investigated in the presence of a stoichiometric amount of 2,4,6-(CH3)3C6H2CO2H as an achiral proton source. The results are summarized in Table 1. Although 2 was slightly more reactive than 1, almost no reaction was observed with 1 and/or 2 even after long reaction times (entries 1 and 2). However, 3–5 gave the desired product with quantitative yield and moderate to good enantioselectivity (entries 3–5). These results again supported our previous hypothesis that introduction of a =NTf group into the phosphoryl group could improve reactivity.6,7 Gratifyingly, we found that substitution of the oxygen with sulfur or selenium in the phosphoramide improved enantioselectivity as well as reactivity (entries 3–5).
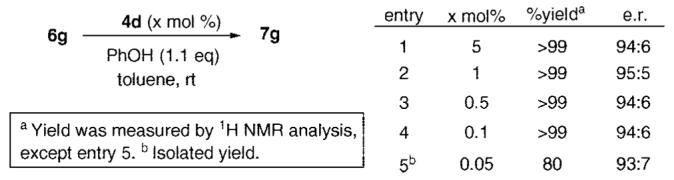
Next, we optimized the reaction conditions for the enantioselective protonation reaction with 4c.14 Among various reaction parameters, we found that reactivities and enantioselectivities of protonation reactions catalyzed by 4c are strongly dependent on the achiral proton sources. Higher enantioselectivity could be achieved with nonhindered phenol and carboxylic acid derivatives as achiral proton sources. Under these optimized conditions, several N-triflyl thiophosphoramides 4a–e with varying substituents at the 3,3′-positions of the binaphthyl scaffold were tested. We found that alkyl substituents at 2,6-positions of aromatic substituents at 3,3′-positions of the binaphthyl scaffold are crucial for enantioselectivity. Enantioselectivity as high as 91:9 enantiomeric ratio (er) could be obtained using 4d as the catalyst.
Using these optimized conditions with catalyst 4d, various silyl enol ethers were studied in the enantioselective protonation reaction. The results are summarized in Table 2. Various 2-aryl-substituted cyclic ketones, bearing either electron-donating or electron-withdrawing substituents, could be obtained in quantitative yields and high enantioselectivities (entries 1–4). However, the enantioselectivity showed a slight dependence on the size of R-substituents of the cyclic ketones. A bulky substituent gave slightly better enantioselectivity (entry 5), while the aryl substituent carrying o-substitution had a deleterious effect on the enantioselectivity (entry 6). In addition, the ring size of the cyclic ketone affected enantioselectivity: the seven-membered ring ketone had better enantioselectivity than six-membered ring ketones (entries 1, 5, 7, and 9). It should be noted that the catalyst loading could be reduced to 1 mol % without any detrimental effect on the selectivity, although longer reaction times were needed (entries 7–10). In addition, asymmetric protonations of the silyl enol ethers of 2-alkyl-substituted cyclic ketones were achieved using this catalytic system in quantitative yields, even though the enantioselectivities were moderate (entries 11 and 12).
Table 2.
Substrate Scope
 | |||||
|---|---|---|---|---|---|
| entry | n | Ar | time (h) | % yielda | erb (config) |
| 1 | 1 | Ph (6a) | 8 | >99 (97) | 91:9 (S) |
| 2 | 1 | 4-MeC6H4 (6b) | 6 | >99 (96) | 93:7 (S) |
| 3 | 1 | 4-MeOC6H4 (6c) | 12 | >99 (98) | 92:8 (S) |
| 4 | 1 | 4-ClC6H4 (6d) | 12 | >99 (95) | 92:8 |
| 5 | 1 | 2-Naphthyl (6e) | 12 | >99 (99) | 93:7 (S) |
| 6c | 1 | 2-MeOC6H4 (6f) | 40 | >99 (97) | 86:14 |
| 7 | 2 | Ph (6g) | 6 | >99 (99) | 94:6 (S) |
| 8c | 2 | Ph (6g) | 24 | >99 | 95:5 (S) |
| 9 | 2 | 2-naphthyl (6h) | 6 | >99 (97) | 95:5 |
| 10c | 2 | 2-naphthyl (6h) | 22 | >99 | 94:6 |
| 11 | 1 | −CH2Ph (6i) (94:6)d | 8 | >99 (97) | 77:23 (S) (79:21)e |
| 12 | 1 | −cyclohexyl (6j) (96:4)d | 8 | >99 (96) | 82:18 (S) (84:16)e |
Yield was measured by 1H NMR analysis, and the isolated yields are shown in parentheses.
Enantiomeric ratio was determined by HPLC or GC analysis.
1 mol % of catalyst was used.
The value in parentheses indicates the regioisomeric ratio of the starting silyl enol ethers.
Corrected value based on the regioisomeric ratio of the starting silyl enol ethers.
With these successful results in terms of catalyst activity and enantioselectivity, we wanted to test the reactivity and robustness of the Brønsted acid by decreasing the catalyst loading. The protonated product of 6g was quantitatively obtained in 94:6 er with a catalyst loading of only 0.1 mol %. Even the smallest substrate/catalyst ratio, namely S/C 2000 at room temperature, provided excellent yield and good enantioselectivity. This was an unprecedented example of chiral phosphoric acid catalysis with such a low catalyst loading, which proves its remarkable catalytic efficiency.15
Preliminary studies into the mechanism of the Brønsted acid catalyzed asymmetric protonation reaction of silyl enol ethers indicate that achiral proton sources play an important role in determining reactivity.14 In the absence of an achiral proton source, even though a stoichiometric amount of chiral Brønsted acid was used, no reaction was observed even after 2 days. However, when the same reaction was carried out in the presence of stoichiometric amount of CH3CO2H as an achiral proton source, the reaction was completed within 2 h with almost the same enantioselectivity as the catalytic one.
These results suggest that the protonation reaction proceeds through a two-step sequence (Scheme 1): initially, the protonation takes place enantioselectively from the chiral Brønsted acid HA or achiral oxonium ion pair [PhOH2]+•A−, generated by rapid proton transfer between HA and the achiral proton source PhOH, to the silyl enol ether a to form an intermediary chiral ion pair [aH]+•[A]−.16 This is followed by the desilylation with PhOH to form the corresponding ketone b, the silylated achiral proton source Ph-OTMS, and the regenerated HA. Without PhOH, the desilylation was very slow because the affinity of the resultant conjugate base [A]− to the silicon is quite low. However, in the presence of PhOH with enough affinity to silicon desilylation could be accelerated.17
Scheme 1.
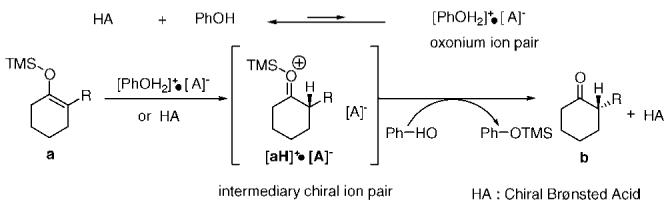
Proposed Mechanism of Brønsted Acid Catalyzed Asymmetric Protonations of Silyl Enol Ethers
In conclusion, we have reported the first metal-free Brønsted acid catalyzed asymmetric protonation reactions of silyl enol ethers using a chiral Brønsted acid catalyst in the presence of achiral Brønsted acid media. In addition, the reactivity of this Brønsted acid is especially appealing for chiral phosphoric acid catalysis in that the catalyst loading for this reaction could be decreased up to 0.05 mol % without any significant loss of enantioselectivity.
Supplementary Material
Acknowledgment
This work was partially supported by NSF (Grant No. 0412060), NIH (Grant No. RO1 GMo74639-01), and Merck. Special thanks to Dr. Ian Steele for X-ray crystallographic analysis.
Footnotes
Supporting Information Available: Experimental procedures and spectroscopic data of new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Pihko PM, Moisan L. Angew. Chem., Int. Ed. 2004;43:2062. For a review of enantioselective organocatalysis, see: see Supporting Information for more references. [Google Scholar]
- 2.Doyle AG, Jacobsen EN. Chem. Rev. 2007;107:5713. doi: 10.1021/cr068373r. For a review of hydrogen bond organic catalysis, see: see Supporting Information for more references. [DOI] [PubMed] [Google Scholar]
- 3.Akiyama T, Itoh J, Yokota D, Fuchibe K. Angew. Chem., Int. Ed. 2004;43:1566. doi: 10.1002/anie.200353240. [DOI] [PubMed] [Google Scholar]
- 4.Uraguchi D, Terada M. J. Am. Chem. Soc. 2004;126:5356. doi: 10.1021/ja0491533. [DOI] [PubMed] [Google Scholar]
- 5.Akiyama T. Chem. Rev. 2007;107:5744. doi: 10.1021/cr068374j. For a review of chiral phosphoric acid catalysis, see: see Supporting Information for more references. [DOI] [PubMed] [Google Scholar]
- 6.(a) Nakashima D, Yamamoto H. J. Am. Chem. Soc. 2006;128:9626. doi: 10.1021/ja062508t. [DOI] [PubMed] [Google Scholar]; (b) Jiao P, Nakashima D, Yamamoto H. Angew. Chem., Int. Ed. 2008;47:2411. doi: 10.1002/anie.200705314. [DOI] [PubMed] [Google Scholar]
- 7.(a) Rueping M, Ieawsuwan D, Antonchick AP, Nachtsheim BJ. Angew. Chem., Int. Ed. 2007;46:2097. doi: 10.1002/anie.200604809. After our publication of N-triflyl phosphamide, two other examples of carbonyl activation with this reagent were published; see: [DOI] [PubMed] [Google Scholar]; (b) Rueping M, Nachtsheim BJ, Moreth SA, Bolte M. Angew. Chem., Int. Ed. 2008;47:593. doi: 10.1002/anie.200703668. [DOI] [PubMed] [Google Scholar]
- 8.Bordwell FG. Acc. Chem. Res. 1988;21:456. [Google Scholar]
- 9.Robak MT, Trincado M, Ellman JA. J. Am. Chem. Soc. 2007;129:15110. doi: 10.1021/ja075653v. Similar acidity enhancement by substitution of the oxygen with sulfur was observed in the urea/thiourea catalyst. See ref 2. [DOI] [PubMed] [Google Scholar]
- 10.Duhamel L, Duhamel P, Plaquevent JC. Tetrahedron: Asymmetry. 2004;15:3653. For a review of enantioselective protonation, see: see Supporting information for more references. [Google Scholar]
- 11.Nakashima D, Yamamoto H. Synlett. 2006:150. For the asymmetric protonation with LBA, see: see Supporting Information for more references. [Google Scholar]
- 12.Yanagisawa A, Touge T, Arai T. Angew. Chem., Int. Ed. 2005;44:1546. doi: 10.1002/anie.200462325. For another example of asymmetric protonation of silyl enol ether with Binap •AgF complex, see: [DOI] [PubMed] [Google Scholar]
- 13.Poisson T, Dalla V, Marsais F, Dupas G, Oudeyer S, Levacher V. Angew. Chem., Int. Ed. 2007;46:7090. doi: 10.1002/anie.200701683. For another excellent organocatalytic asymmetric protonation of silyl enol ethers in different systems was reported, see: [DOI] [PubMed] [Google Scholar]
- 14.See Supporting Information for more detailed experimental results.
- 15.Terada M, Machioka K, Sorimachi K. Angew. Chem., Int. Ed. 2006;45:2254. doi: 10.1002/anie.200503477. For another excellent example of high S/C ratio with phosphoric acid, see: [DOI] [PubMed] [Google Scholar]
- 16.(a) Mayer S, List B. Angew. Chem., Int. Ed. 2005;45:4193. doi: 10.1002/anie.200600512. For a concept of asymmetric counteranion-directed catalysis, see: [DOI] [PubMed] [Google Scholar]; (b) Hamilton GL, Kang EJ, Mba M, Toste FD. Science. 2007;317:496. doi: 10.1126/science.1145229. [DOI] [PubMed] [Google Scholar]
- 17.Sickert M, Schneider C. Angew. Chem., Int. Ed. 2008;47:3631. doi: 10.1002/anie.200800103. Similar reaction rate enhancement of phosphoric acid catalysis in alcoholic solvents: [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


