Abstract
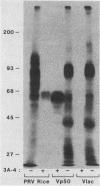
Pseudorabies virus (PRV) is an alphaherpesvirus which causes an economically important disease of swine. One of the PRV glycoproteins, gp50, was previously identified as the sequence homolog of herpes simplex virus glycoprotein gD (E.A. Petrovskis, J.G. Timmins, M.A. Armentrout, C.C. Marchioli, R.J. Yancey, Jr., and L.E. Post, J. Virol. 59:216-223, 1986). gp50 was evaluated as a PRV subunit vaccine candidate. gp50 protected mice from PRV-induced mortality either when delivered via infection with a recombinant vaccinia virus or when administered as a subunit vaccine produced in a eucaryotic cell line, Chinese hamster ovary (CHO) cells. In addition, gp50 synthesized in CHO cells protected pigs from lethal infection with PRV. This result demonstrates that a single viral glycoprotein could induce a protective immune response in the natural host of a herpesvirus infection.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banks M., Cartwright S. Comparison and evaluation of four serological tests for detection of antibodies to Aujeszky's disease virus. Vet Rec. 1983 Jul 9;113(2):38–41. doi: 10.1136/vr.113.2.38. [DOI] [PubMed] [Google Scholar]
- Berman P. W., Gregory T., Crase D., Lasky L. A. Protection from genital herpes simplex virus type 2 infection by vaccination with cloned type 1 glycoprotein D. Science. 1985 Mar 22;227(4693):1490–1492. doi: 10.1126/science.2983428. [DOI] [PubMed] [Google Scholar]
- Brown F., Schild G. C., Ada G. L. Recombinant vaccinia viruses as vaccines. Nature. 1986 Feb 13;319(6054):549–550. doi: 10.1038/319549a0. [DOI] [PubMed] [Google Scholar]
- Buchman T. G., Roizman B., Nahmias A. J. Demonstration of exogenous genital reinfection with herpes simplex virus type 2 by restriction endonuclease fingerprinting of viral DNA. J Infect Dis. 1979 Sep;140(3):295–304. doi: 10.1093/infdis/140.3.295. [DOI] [PubMed] [Google Scholar]
- Chan W. L. Protective immunization of mice with specific HSV-1 glycoproteins. Immunology. 1983 Jun;49(2):343–352. [PMC free article] [PubMed] [Google Scholar]
- Cohen G. H., Katze M., Hydrean-Stern C., Eisenberg R. J. Type-common CP-1 antigen of herpes simplex virus is associated with a 59,000-molecular-weight envelope glycoprotein. J Virol. 1978 Jul;27(1):172–181. doi: 10.1128/jvi.27.1.172-181.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremer K. J., Mackett M., Wohlenberg C., Notkins A. L., Moss B. Vaccinia virus recombinant expressing herpes simplex virus type 1 glycoprotein D prevents latent herpes in mice. Science. 1985 May 10;228(4700):737–740. doi: 10.1126/science.2986288. [DOI] [PubMed] [Google Scholar]
- Dix R. D., Mills J. Acute and latent herpes simplex virus neurological disease in mice immunized with purified virus-specific glycoproteins gB or gD. J Med Virol. 1985 Sep;17(1):9–18. doi: 10.1002/jmv.1890170103. [DOI] [PubMed] [Google Scholar]
- Eisenberg R. J., Cerini C. P., Heilman C. J., Joseph A. D., Dietzschold B., Golub E., Long D., Ponce de Leon M., Cohen G. H. Synthetic glycoprotein D-related peptides protect mice against herpes simplex virus challenge. J Virol. 1985 Dec;56(3):1014–1017. doi: 10.1128/jvi.56.3.1014-1017.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long D., Madara T. J., Ponce de Leon M., Cohen G. H., Montgomery P. C., Eisenberg R. J. Glycoprotein D protects mice against lethal challenge with herpes simplex virus types 1 and 2. Infect Immun. 1984 Feb;43(2):761–764. doi: 10.1128/iai.43.2.761-764.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackett M., Smith G. L., Moss B. General method for production and selection of infectious vaccinia virus recombinants expressing foreign genes. J Virol. 1984 Mar;49(3):857–864. doi: 10.1128/jvi.49.3.857-864.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackett M., Smith G. L. Vaccinia virus expression vectors. J Gen Virol. 1986 Oct;67(Pt 10):2067–2082. doi: 10.1099/0022-1317-67-10-2067. [DOI] [PubMed] [Google Scholar]
- Mackett M., Yilma T., Rose J. K., Moss B. Vaccinia virus recombinants: expression of VSV genes and protective immunization of mice and cattle. Science. 1985 Jan 25;227(4685):433–435. doi: 10.1126/science.2981435. [DOI] [PubMed] [Google Scholar]
- Maes R. K., Schutz J. C. Evaluation in swine of a subunit vaccine against pseudorabies. Am J Vet Res. 1983 Jan;44(1):123–125. [PubMed] [Google Scholar]
- Martin S., Moss B., Berman P. W., Laskey L. A., Rouse B. T. Mechanisms of antiviral immunity induced by a vaccinia virus recombinant expressing herpes simplex virus type 1 glycoprotein D: cytotoxic T cells. J Virol. 1987 Mar;61(3):726–734. doi: 10.1128/jvi.61.3.726-734.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick F., Trahey M., Innis M., Dieckmann B., Ringold G. Inducible expression of amplified human beta interferon genes in CHO cells. Mol Cell Biol. 1984 Jan;4(1):166–172. doi: 10.1128/mcb.4.1.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeoch D. J., Moss H. W., McNab D., Frame M. C. DNA sequence and genetic content of the HindIII l region in the short unique component of the herpes simplex virus type 2 genome: identification of the gene encoding glycoprotein G, and evolutionary comparisons. J Gen Virol. 1987 Jan;68(Pt 1):19–38. doi: 10.1099/0022-1317-68-1-19. [DOI] [PubMed] [Google Scholar]
- Mettenleiter T. C., Lukacs N., Rziha H. J. Mapping of the structural gene of pseudorabies virus glycoprotein A and identification of two non-glycosylated precursor polypeptides. J Virol. 1985 Jan;53(1):52–57. doi: 10.1128/jvi.53.1.52-57.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti E., Lipinskas B. R., Samsonoff C., Mercer S., Panicali D. Construction of live vaccines using genetically engineered poxviruses: biological activity of vaccinia virus recombinants expressing the hepatitis B virus surface antigen and the herpes simplex virus glycoprotein D. Proc Natl Acad Sci U S A. 1984 Jan;81(1):193–197. doi: 10.1073/pnas.81.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Para M. F., Parish M. L., Noble A. G., Spear P. G. Potent neutralizing activity associated with anti-glycoprotein D specificity among monoclonal antibodies selected for binding to herpes simplex virions. J Virol. 1985 Aug;55(2):483–488. doi: 10.1128/jvi.55.2.483-488.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovskis E. A., Timmins J. G., Armentrout M. A., Marchioli C. C., Yancey R. J., Jr, Post L. E. DNA sequence of the gene for pseudorabies virus gp50, a glycoprotein without N-linked glycosylation. J Virol. 1986 Aug;59(2):216–223. doi: 10.1128/jvi.59.2.216-223.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovskis E. A., Timmins J. G., Post L. E. Use of lambda gt11 to isolate genes for two pseudorabies virus glycoproteins with homology to herpes simplex virus and varicella-zoster virus glycoproteins. J Virol. 1986 Oct;60(1):185–193. doi: 10.1128/jvi.60.1.185-193.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt K. B. The evaluation of a lectin-agarose based subunit vaccine and complementary diagnostic antigen for Aujeszky's disease (pseudorabies) in the pig. Vet Microbiol. 1984 Feb;9(1):35–51. doi: 10.1016/0378-1135(84)90077-4. [DOI] [PubMed] [Google Scholar]
- Platt K. B. The porcine humoral response to detergent extracted Aujeszky's disease (pseudorabies) virus antigens. Vet Microbiol. 1982 Dec;7(6):515–534. doi: 10.1016/0378-1135(82)90046-3. [DOI] [PubMed] [Google Scholar]
- Poorman R. A., Palermo D. P., Post L. E., Murakami K., Kinner J. H., Smith C. W., Reardon I., Heinrikson R. L. Isolation and characterization of native human renin derived from Chinese hamster ovary cells. Proteins. 1986 Oct;1(2):139–145. doi: 10.1002/prot.340010206. [DOI] [PubMed] [Google Scholar]
- Rea T. J., Timmins J. G., Long G. W., Post L. E. Mapping and sequence of the gene for the pseudorabies virus glycoprotein which accumulates in the medium of infected cells. J Virol. 1985 Apr;54(1):21–29. doi: 10.1128/jvi.54.1.21-29.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins A. K., Dorney D. J., Wathen M. W., Whealy M. E., Gold C., Watson R. J., Holland L. E., Weed S. D., Levine M., Glorioso J. C. The pseudorabies virus gII gene is closely related to the gB glycoprotein gene of herpes simplex virus. J Virol. 1987 Sep;61(9):2691–2701. doi: 10.1128/jvi.61.9.2691-2701.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins A. K., Watson R. J., Whealy M. E., Hays W. W., Enquist L. W. Characterization of a pseudorabies virus glycoprotein gene with homology to herpes simplex virus type 1 and type 2 glycoprotein C. J Virol. 1986 May;58(2):339–347. doi: 10.1128/jvi.58.2.339-347.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrier R. D., Pizer L. I., Moorhead J. W. Type-specific delayed hypersensitivity and protective immunity induced by isolated herpes simplex virus glycoprotein. J Immunol. 1983 Mar;130(3):1413–1418. [PubMed] [Google Scholar]
- Torseth J. W., Cohen G. H., Eisenberg R. J., Berman P. W., Lasky L. A., Cerini C. P., Heilman C. J., Kerwar S., Merigan T. C. Native and recombinant herpes simplex virus type 1 envelope proteins induce human immune T-lymphocyte responses. J Virol. 1987 May;61(5):1532–1539. doi: 10.1128/jvi.61.5.1532-1539.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner S. P., Hartley C. E., Buchan A., Skinner G. R. Preparation and efficacy of an inactivated subunit vaccine against Aujeszky's disease virus infection. Res Vet Sci. 1981 Sep;31(2):261–263. [PubMed] [Google Scholar]
- Watari E., Dietzschold B., Szokan G., Heber-Katz E. A synthetic peptide induces long-term protection from lethal infection with herpes simplex virus 2. J Exp Med. 1987 Feb 1;165(2):459–470. doi: 10.1084/jem.165.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wathen L. M., Platt K. B., Wathen M. W., Van Deusen R. A., Whetstone C. A., Pirtle E. C. Production and characterization of monoclonal antibodies directed against pseudorabies virus. Virus Res. 1985 Dec;4(1):19–29. doi: 10.1016/0168-1702(85)90017-6. [DOI] [PubMed] [Google Scholar]
- Wathen M. W., Wathen L. M. Isolation, characterization, and physical mapping of a pseudorabies virus mutant containing antigenically altered gp50. J Virol. 1984 Jul;51(1):57–62. doi: 10.1128/jvi.51.1.57-62.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarling J. M., Moran P. A., Burke R. L., Pachl C., Berman P. W., Lasky L. A. Human cytotoxic T cell clones directed against herpes simplex virus-infected cells. IV. Recognition and activation by cloned glycoproteins gB and gD. J Immunol. 1986 Jun 15;136(12):4669–4673. [PubMed] [Google Scholar]
- Zarling J. M., Moran P. A., Lasky L. A., Moss B. Herpes simplex virus (HSV)-specific human T-cell clones recognize HSV glycoprotein D expressed by a recombinant vaccinia virus. J Virol. 1986 Aug;59(2):506–509. doi: 10.1128/jvi.59.2.506-509.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leeuw P. W., van Oirschot J. T. Vaccines against Aujeszky's disease: evaluation of their efficacy under standardized laboratory conditions. Vet Q. 1985 Jul;7(3):191–197. doi: 10.1080/01652176.1985.9693982. [DOI] [PubMed] [Google Scholar]