Abstract
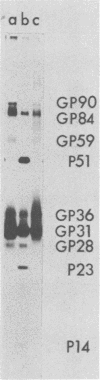
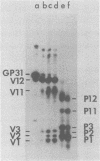
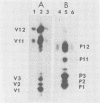
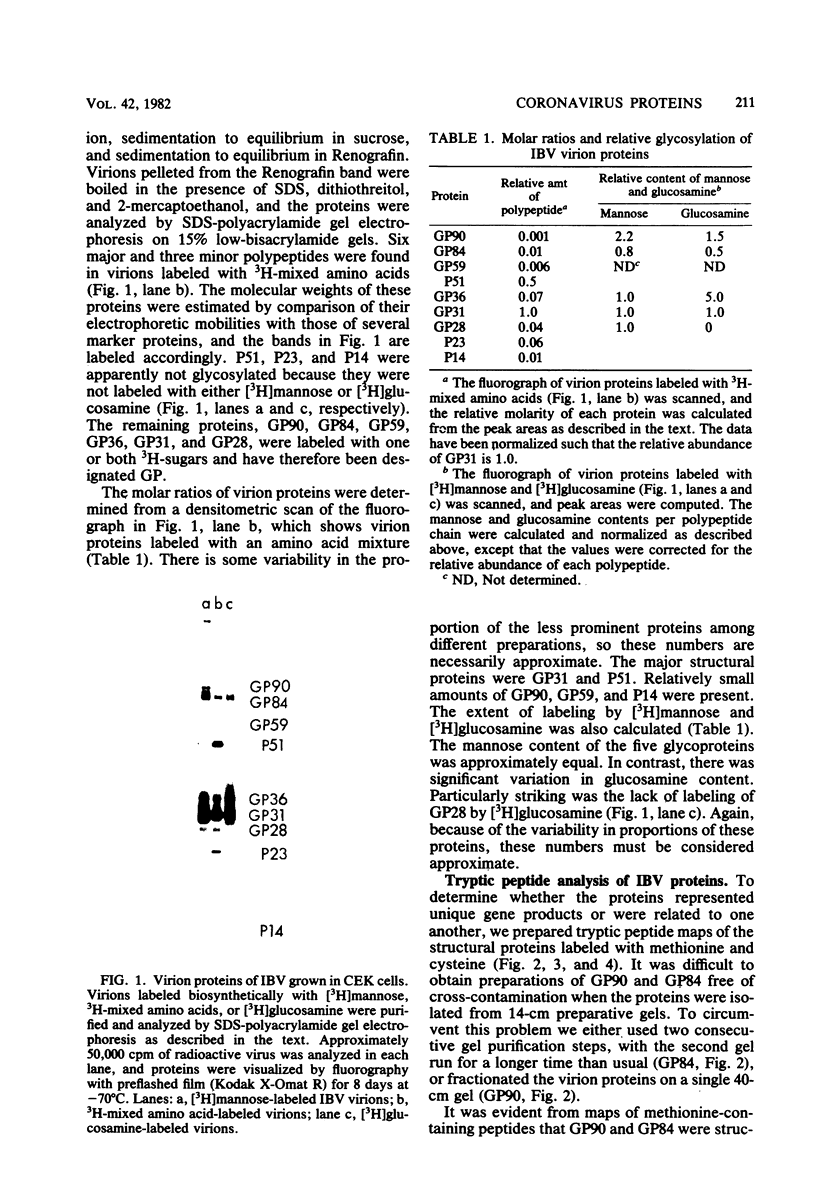
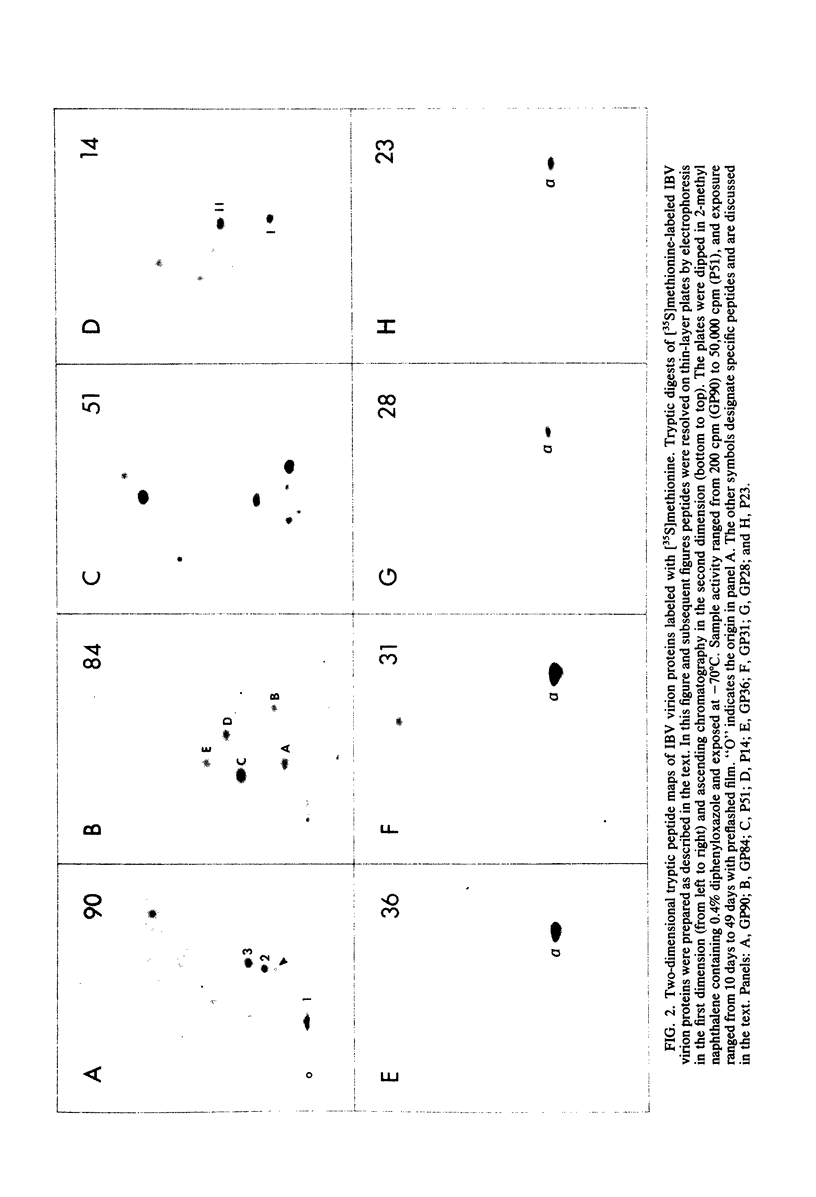
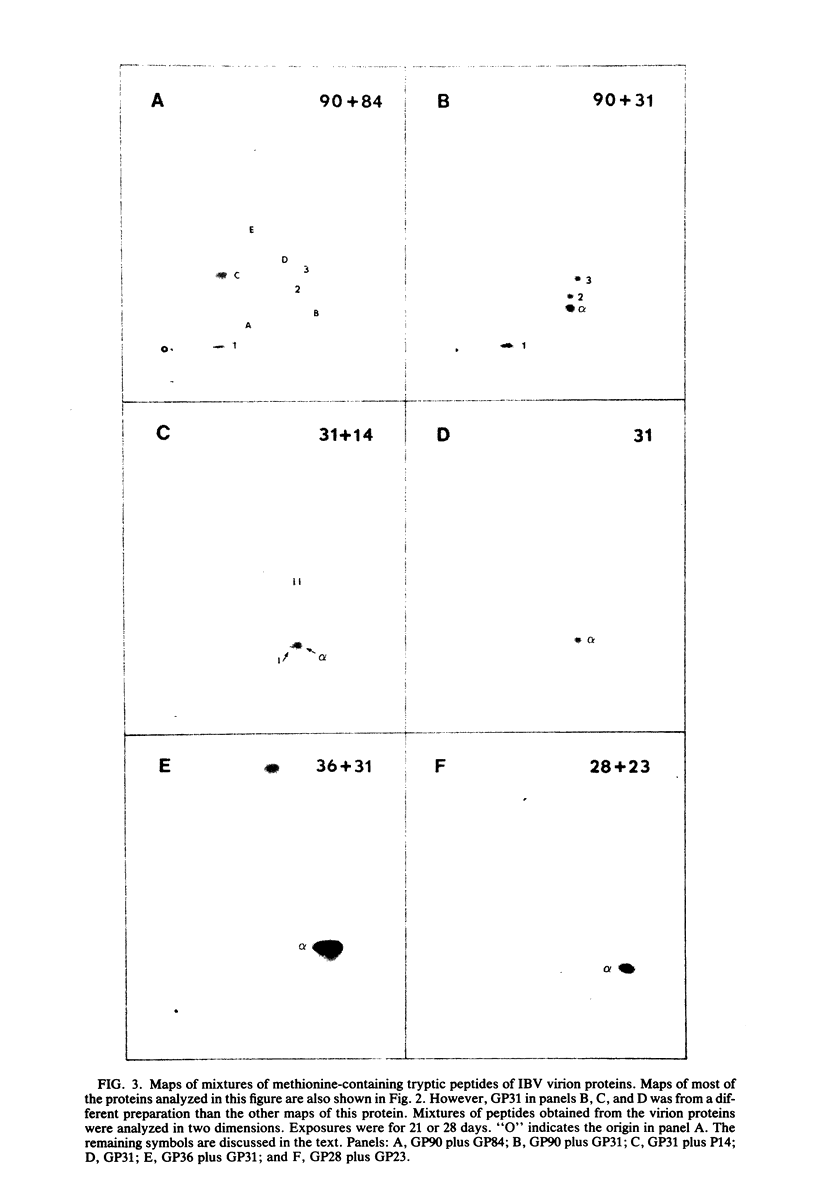
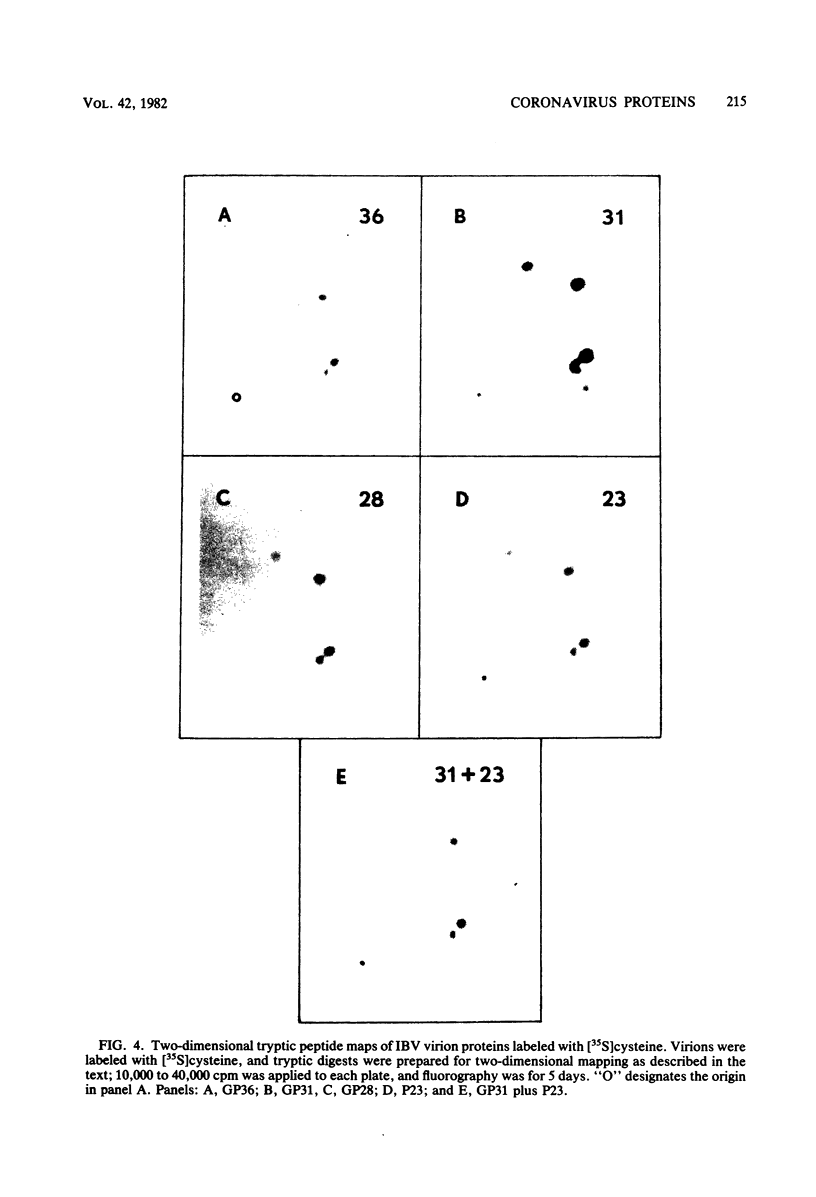
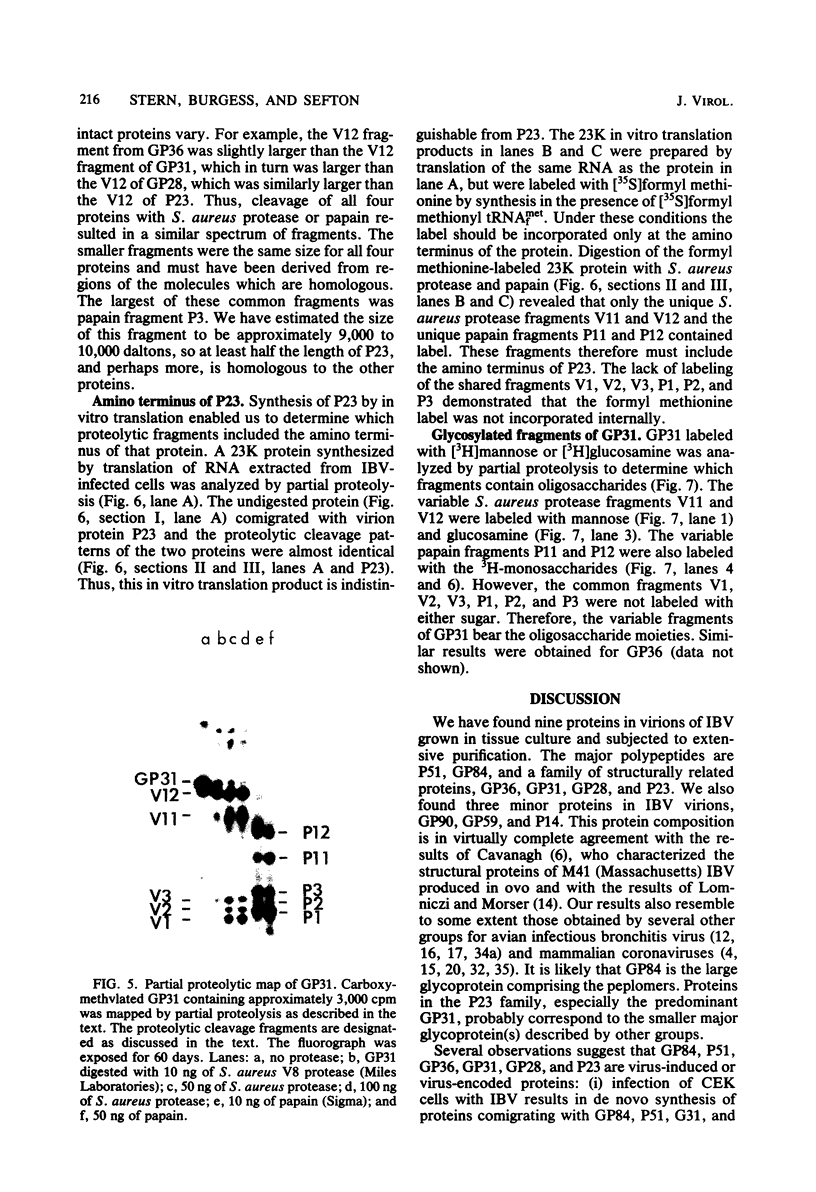
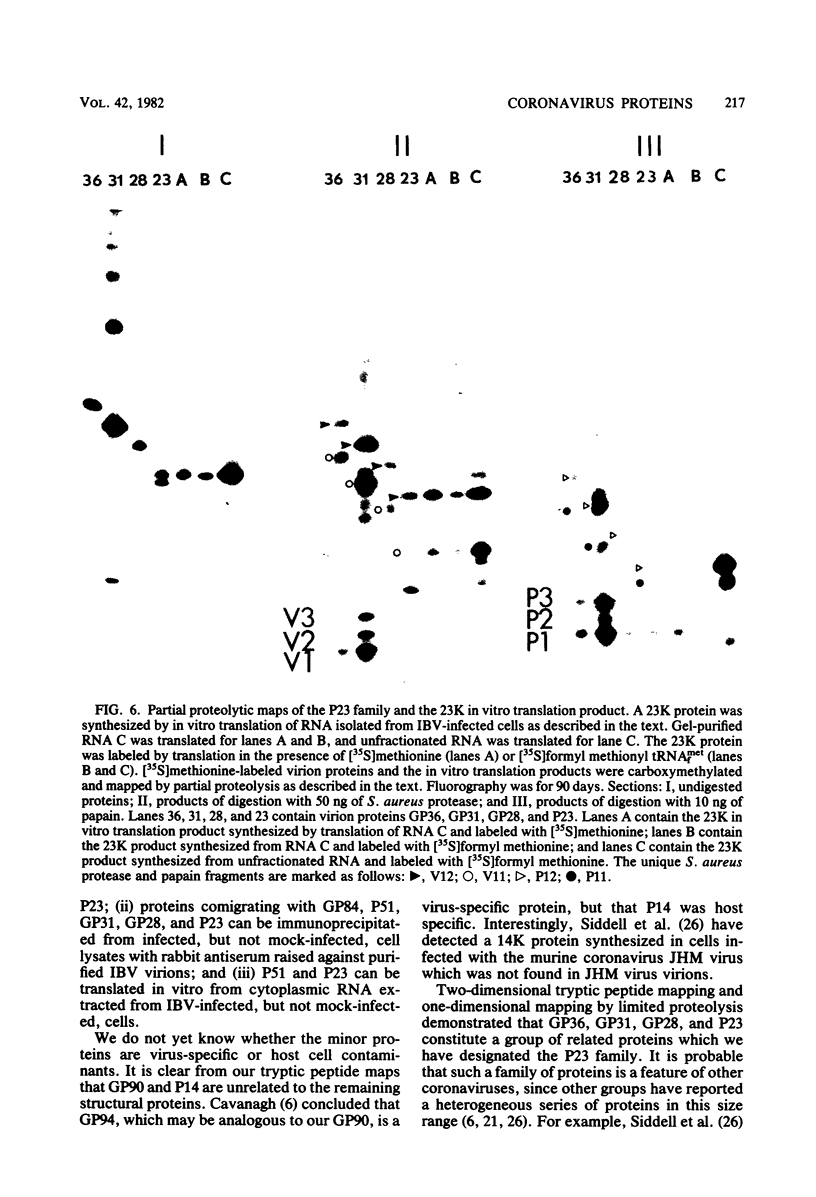
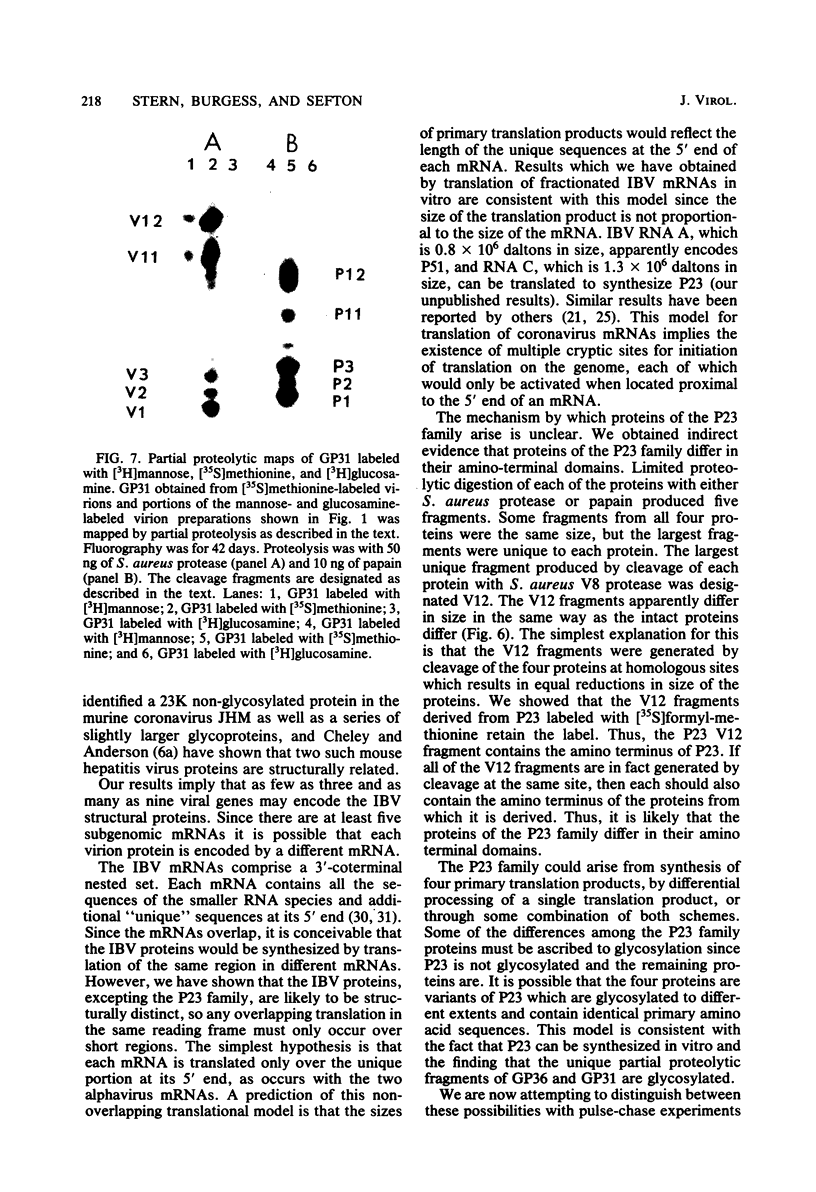
We have found six major polypeptides in virions of the avian coronavirus infectious bronchitis virus grown in tissue culture: four glycoproteins, GP84, GP36, GP31, and GP28, and two non-glycosylated proteins, P51 and P23. In addition, we detected three minor species: two glycoproteins, GP90 and GP59, and one non-glycosylated protein, P14. Two-dimensional tryptic peptide mapping showed that GP36, GP31, GP28, and P23 comprise a group of closely related proteins which we have designated the "P23 family," but that the other proteins are distinct. Analysis by partial proteolytic digestion of P23 family, but that the other proteins are distinct. Analysis by partial proteolytic digestion of the P23 family labeled biosynthetically with [35S] methionine, and P23, labeled with [35S] formyl-methionine by in vitro translation of RNA from infected cells, revealed that the proteins of the P23 family differ in their amino-terminal domains. Similar analysis of GP31 and Gp36 labeled with [3H] mannose showed that the partial proteolytic fragments unique to these proteins were glycosylated. This suggests that differences in glycosylation in the amino-terminal domains contributes to the marked polymorphism os the P23 family. The results are discussed with respect to possible models for synthesis of the virion proteins.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beemon K., Hunter T. Characterization of Rous sarcoma virus src gene products synthesized in vitro. J Virol. 1978 Nov;28(2):551–566. doi: 10.1128/jvi.28.2.551-566.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingham R. W. The polypeptide composition of avian infectious bronchitis virus. Arch Virol. 1975;49(2-3):207–216. doi: 10.1007/BF01317539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonatti S., Sonenberg N., Shatkin A. J., Cancedda R. Restricted initiation of protein synthesis on the potentially polycistronic Sindbis virus 42 S RNA. J Biol Chem. 1980 Dec 10;255(23):11473–11477. [PubMed] [Google Scholar]
- Bond C. W., Leibowitz J. L., Robb J. A. Pathogenic murine coronaviruses. II. Characterization of virus-specific proteins of murine coronaviruses JHMV and A59V. Virology. 1979 Apr 30;94(2):371–384. doi: 10.1016/0042-6822(79)90468-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Stedman J. D. Efficient fluorography of 3H and 14C on thin layers. Anal Biochem. 1978 Aug 15;89(1):247–256. doi: 10.1016/0003-2697(78)90747-9. [DOI] [PubMed] [Google Scholar]
- Cavanagh D. Structural polypeptides of coronavirus IBV. J Gen Virol. 1981 Mar;53(Pt 1):93–103. doi: 10.1099/0022-1317-53-1-93. [DOI] [PubMed] [Google Scholar]
- Cheley S., Anderson R. Cellular synthesis and modification of murine hepatitis virus polypeptides. J Gen Virol. 1981 Jun;54(Pt 2):301–311. doi: 10.1099/0022-1317-54-2-301. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Collins M. S., Alexander D. J. Avian infectious bronchitis virus structural polypeptides: effect of different conditions of disruption and comparison of different strains and isolates. Arch Virol. 1980;63(3-4):239–251. doi: 10.1007/BF01315030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Garrels J. I. Characterization of the mRNAs for alpha-, beta- and gamma-actin. Cell. 1977 Nov;12(3):767–781. doi: 10.1016/0092-8674(77)90276-8. [DOI] [PubMed] [Google Scholar]
- Kitamura N., Semler B. L., Rothberg P. G., Larsen G. R., Adler C. J., Dorner A. J., Emini E. A., Hanecak R., Lee J. J., van der Werf S. Primary structure, gene organization and polypeptide expression of poliovirus RNA. Nature. 1981 Jun 18;291(5816):547–553. doi: 10.1038/291547a0. [DOI] [PubMed] [Google Scholar]
- Lanser J. A., Howard C. R. The polypeptides of infectious bronchitis virus (IBV-41 strain). J Gen Virol. 1980 Feb;46(2):349–361. doi: 10.1099/0022-1317-46-2-349. [DOI] [PubMed] [Google Scholar]
- Leibowitz J. L., Wilhelmsen K. C., Bond C. W. The virus-specific intracellular RNA species of two murine coronaviruses: MHV-a59 and MHV-JHM. Virology. 1981 Oct 15;114(1):39–51. doi: 10.1016/0042-6822(81)90250-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomniczi B., Morser J. Polypeptides of infectious bronchitis virus. I. Polypeptides of the virion. J Gen Virol. 1981 Jul;55(Pt 1):155–164. doi: 10.1099/0022-1317-55-1-155. [DOI] [PubMed] [Google Scholar]
- Macnaughton M. R., Madge M. H. The polypeptide composition of avain infectious bronchitis virus particles. Arch Virol. 1977;55(1-2):47–54. doi: 10.1007/BF01314478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macnaughton M. R. The polypeptides of human and mouse coronaviruses. Brief report. Arch Virol. 1980;63(1):75–80. doi: 10.1007/BF01320763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy E., Lomniczi B. Polypeptide patterns of infectious bronchitis virus serotypes fall into two categories. Brief report. Arch Virol. 1979;61(4):341–345. doi: 10.1007/BF01315022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papkoff J., Hunter T., Beemon K. In vitro translation of virion RNA from Moloney murine sarcoma virus. Virology. 1980 Feb;101(1):91–103. doi: 10.1016/0042-6822(80)90486-9. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Rottier P. J., Spaan W. J., Horzinek M. C., van der Zeijst B. A. Translation of three mouse hepatitis virus strain A59 subgenomic RNAs in Xenopus laevis oocytes. J Virol. 1981 Apr;38(1):20–26. doi: 10.1128/jvi.38.1.20-26.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield P., Zamecnik P. C. Cupric ion catalysis in hydrolysis of aminoacyl-tRNA. Biochim Biophys Acta. 1968 Feb 26;155(2):410–416. doi: 10.1016/0005-2787(68)90185-8. [DOI] [PubMed] [Google Scholar]
- Sefton B. M., Beemon K., Hunter T. Comparison of the expression of the src gene of Rous sarcoma virus in vitro and in vivo. J Virol. 1978 Dec;28(3):957–971. doi: 10.1128/jvi.28.3.957-971.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddell S. G., Barthel A., ter Meulen V. Coronavirus JHM: a virion-associated protein kinase. J Gen Virol. 1981 Feb;52(Pt 2):235–243. doi: 10.1099/0022-1317-52-2-235. [DOI] [PubMed] [Google Scholar]
- Siddell S. G., Wege H., Barthel A., ter Meulen V. Coronavirus JHM: cell-free synthesis of structural protein p60. J Virol. 1980 Jan;33(1):10–17. doi: 10.1128/jvi.33.1.10-17.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddell S., Wege H., Barthel A., ter Meulen V. Coronavirus JHM: intracellular protein synthesis. J Gen Virol. 1981 Mar;53(Pt 1):145–155. doi: 10.1099/0022-1317-53-1-145. [DOI] [PubMed] [Google Scholar]
- Simmons D. T., Strauss J. H. Translation of Sindbis virus 26 S RNA and 49 S RNA in lysates of rabbit reticulocytes. J Mol Biol. 1974 Jun 25;86(2):397–409. doi: 10.1016/0022-2836(74)90027-8. [DOI] [PubMed] [Google Scholar]
- Spaan W. J., Rottier P. J., Horzinek M. C., van der Zeijst B. A. Isolation and identification of virus-specific mRNAs in cells infected with mouse hepatitis virus (MHV-A59). Virology. 1981 Jan 30;108(2):424–434. doi: 10.1016/0042-6822(81)90449-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley W. M., Jr Preparation and analysis of L-( 35 S)methionine labeled transfer ribonucleic acids from rabbit liver. Anal Biochem. 1972 Jul;48(1):202–216. doi: 10.1016/0003-2697(72)90183-2. [DOI] [PubMed] [Google Scholar]
- Stern D. F., Kennedy S. I. Coronavirus multiplication strategy. I. Identification and characterization of virus-specified RNA. J Virol. 1980 Jun;34(3):665–674. doi: 10.1128/jvi.34.3.665-674.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern D. F., Kennedy S. I. Coronavirus multiplication strategy. II. Mapping the avian infectious bronchitis virus intracellular RNA species to the genome. J Virol. 1980 Nov;36(2):440–449. doi: 10.1128/jvi.36.2.440-449.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturman L. S., Holmes K. V., Behnke J. Isolation of coronavirus envelope glycoproteins and interaction with the viral nucleocapsid. J Virol. 1980 Jan;33(1):449–462. doi: 10.1128/jvi.33.1.449-462.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturman L. S., Holmes K. V. Characterization of coronavirus II. Glycoproteins of the viral envelope: tryptic peptide analysis. Virology. 1977 Apr;77(2):650–660. doi: 10.1016/0042-6822(77)90489-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturman L. S. I. Structural proteins: effects of preparative conditions on the migration of protein in polyacrylamide gels. Virology. 1977 Apr;77(2):637–649. doi: 10.1016/0042-6822(77)90488-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadey C. N., Westaway E. G. Structural proteins and glycoproteins of infectious bronchitis virus particles labeled during growth in chick embryo cells. Intervirology. 1981;15(1):19–27. doi: 10.1159/000149210. [DOI] [PubMed] [Google Scholar]
- Wege H., Wege H., Nagashima K., ter Meulen V. Structural polypeptides of the murine coronavirus JHM. J Gen Virol. 1979 Jan;42(1):37–47. doi: 10.1099/0022-1317-42-1-37. [DOI] [PubMed] [Google Scholar]
- Welch W. J., Sefton B. M., Esch F. S. Amino-terminal sequence analysis of alphavirus polypeptides. J Virol. 1981 Jun;38(3):968–972. doi: 10.1128/jvi.38.3.968-972.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]