Abstract
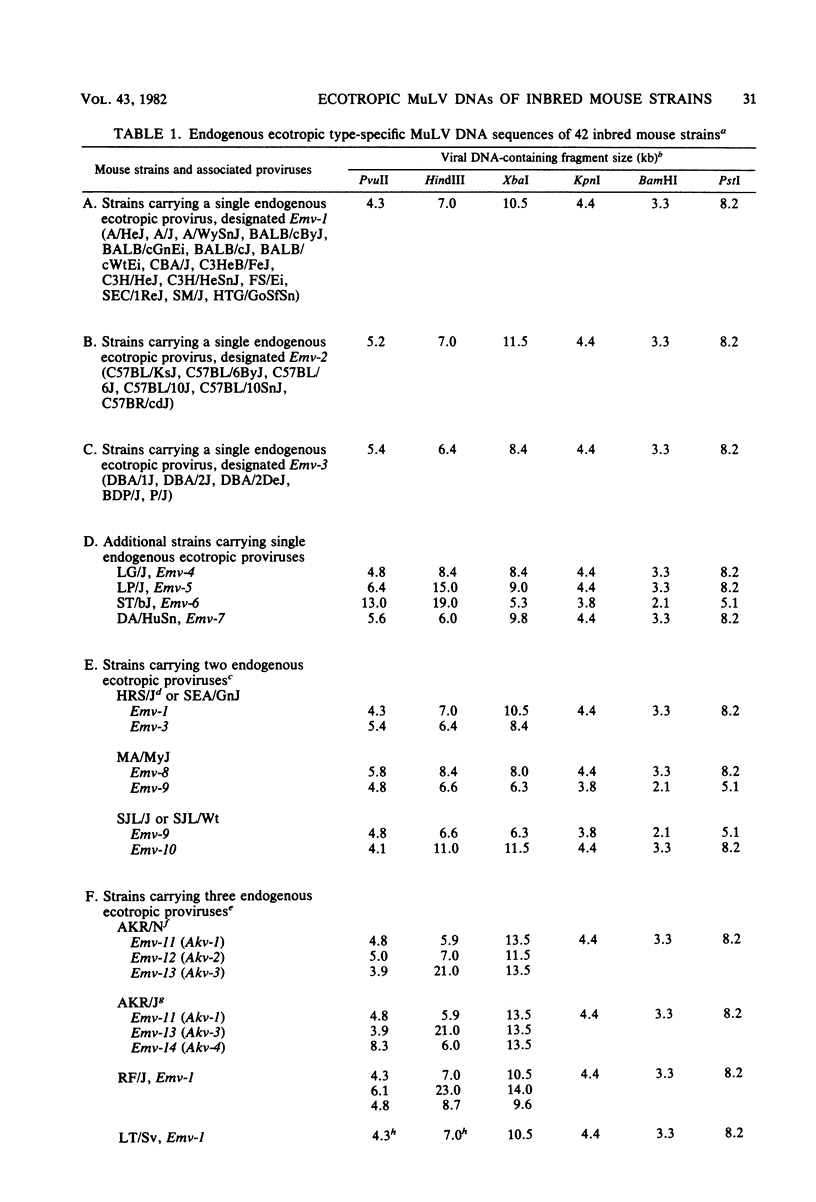
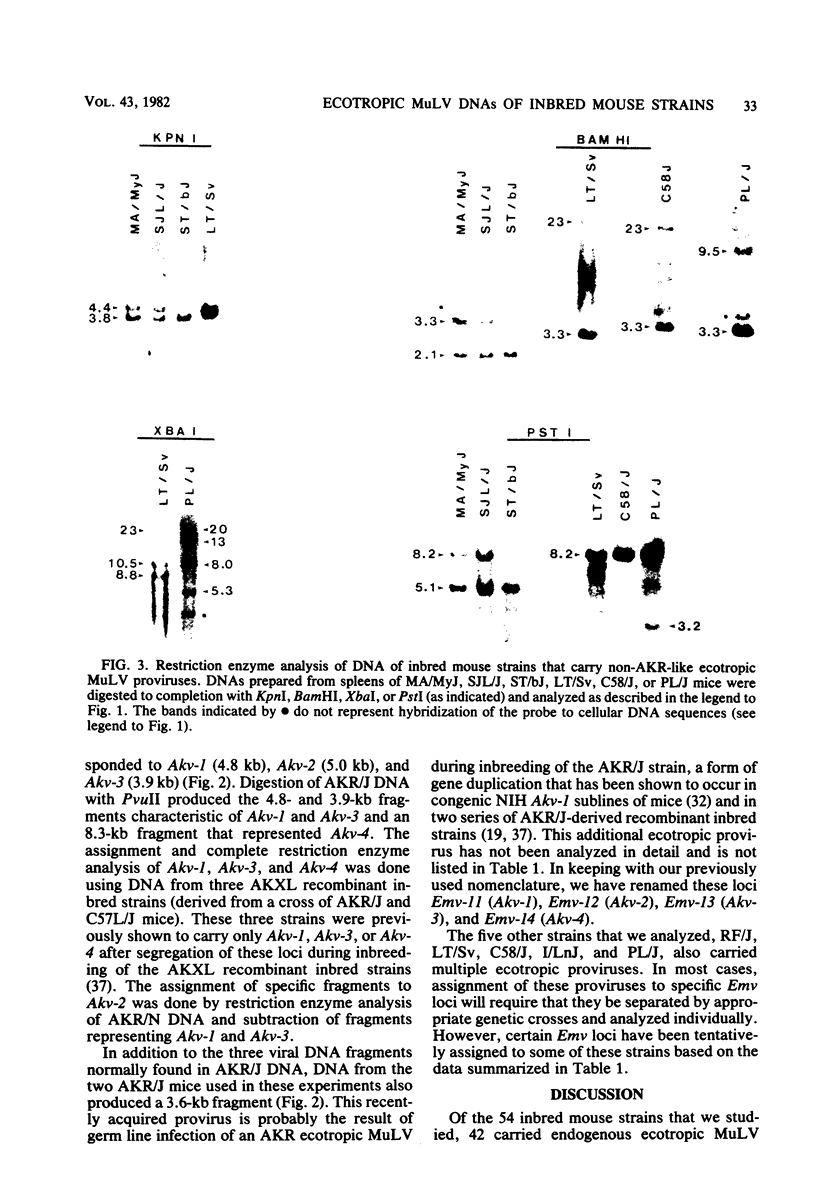
The endogenous ecotropic murine leukemia virus DNA content and integration sites were characterized for 54 inbred strains and substrains of mice by restriction enzyme digestion, Southern blotting, and hybridization with an ecotropic murine leukemia virus DNA-specific probe. More than 75% of these strains carried endogenous ecotropic proviruses which were located in at least 29 distinct integration sites in chromosomes of Mus musculus. Fourteen of these proviruses have been assigned specific locus designations. Most, but not all, of the endogenous ecotropic proviruses were structurally indistinguishable by this analysis from the prototype AKR ecotropic virus, and the distribution of these proviruses followed known relationships among the inbred strains and substrains of mice. These results suggest that, in general, viral DNA integration preceded the establishment of inbred mouse strains and that these integrations are relatively stable.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Astrin S. M. Endogenous viral genes of the White Leghorn chicken: common site of residence and sites associated with specific phenotypes of viral gene expression. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5941–5945. doi: 10.1073/pnas.75.12.5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astrin S. M., Robinson H. L., Crittenden L. B., Buss E. G., Wyban J., Hayward W. S. Ten genetic loci in the chicken that contain structural genes for endogenous avian leukosis viruses. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):1105–1109. doi: 10.1101/sqb.1980.044.01.119. [DOI] [PubMed] [Google Scholar]
- Callahan R., Benveniste R. E., Lieber M. M., Todaro G. J. Nucleic acid homology of murine type-C viral genes. J Virol. 1974 Dec;14(6):1394–1403. doi: 10.1128/jvi.14.6.1394-1403.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canaani E., Aaronson S. A. Restriction enzyme analysis of mouse cellular type C viral DNA: emergence of new viral sequences in spontaneous AKR/J lymphomas. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1677–1681. doi: 10.1073/pnas.76.4.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Lander M. R., Rands E., Lowy D. R. Structure of endogenous murine leukemia virus DNA in mouse genomes. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5774–5778. doi: 10.1073/pnas.77.10.5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Lander M. R., Rowe W. P. Close similarity between endogenous ecotropic virus of Mus musculus molossinus and AKR virus. J Virol. 1980 Nov;36(2):499–505. doi: 10.1128/jvi.36.2.499-505.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland N. G., Zelenetz A. D., Cooper G. M. Transformation of NIH/3T3 mouse cells by DNA of Rous sarcoma virus. Cell. 1979 Aug;17(4):993–1002. doi: 10.1016/0092-8674(79)90338-6. [DOI] [PubMed] [Google Scholar]
- Dhar R., McClements W. L., Enquist L. W., Vande Woude G. F. Nucleotide sequences of integrated Moloney sarcoma provirus long terminal repeats and their host and viral junctions. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3937–3941. doi: 10.1073/pnas.77.7.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farabaugh P. J., Fink G. R. Insertion of the eukaryotic transposable element Ty1 creates a 5-base pair duplication. Nature. 1980 Jul 24;286(5771):352–356. doi: 10.1038/286352a0. [DOI] [PubMed] [Google Scholar]
- Flavell A. J., Ish-Horowicz D. Extrachromosomal circular copies of the eukaryotic transposable element copia in cultured Drosophila cells. Nature. 1981 Aug 13;292(5824):591–595. doi: 10.1038/292591a0. [DOI] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P. Naturally occurring murine leukemia viruses in wild mice: characterization of a new "amphotropic" class. J Virol. 1976 Jul;19(1):19–25. doi: 10.1128/jvi.19.1.19-25.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward W. S., Neel B. G., Astrin S. M. Activation of a cellular onc gene by promoter insertion in ALV-induced lymphoid leukosis. Nature. 1981 Apr 9;290(5806):475–480. doi: 10.1038/290475a0. [DOI] [PubMed] [Google Scholar]
- Hiai H., Morrissey P., Khiroya R., Schwartz R. S. Selective expression of xenotropic virus in congenic HRS/J (hairless) mice. Nature. 1977 Nov 17;270(5634):247–249. doi: 10.1038/270247a0. [DOI] [PubMed] [Google Scholar]
- Hishinuma F., DeBona P. J., Astrin S., Skalka A. M. Nucleotide sequence of acceptor site and termini of integrated avian endogenous provirus ev1: integration creates a 6 bp repeat of host DNA. Cell. 1981 Jan;23(1):155–164. doi: 10.1016/0092-8674(81)90280-4. [DOI] [PubMed] [Google Scholar]
- Ihle J. N., Joseph D. R., Domotor J. J., Jr Genetic linkage of C3H/HeJ and BALB/c endogenous ecotropic C-type viruses to phosphoglucomutase-1 on chromosome 5. Science. 1979 Apr 6;204(4388):71–73. doi: 10.1126/science.219476. [DOI] [PubMed] [Google Scholar]
- Ihle J. N., Joseph D. R. Serological and virological analysis of NIH (NIH X AKR) mice: evidence for three AKR murine leukemia virus loci. Virology. 1978 Jun 15;87(2):287–297. doi: 10.1016/0042-6822(78)90134-4. [DOI] [PubMed] [Google Scholar]
- Jenkins N. A., Copeland N. G., Taylor B. A., Lee B. K. Dilute (d) coat colour mutation of DBA/2J mice is associated with the site of integration of an ecotropic MuLV genome. Nature. 1981 Oct 1;293(5831):370–374. doi: 10.1038/293370a0. [DOI] [PubMed] [Google Scholar]
- Kozak C. A., Rowe W. P. Genetic mapping of the ecotropic murine leukemia virus-inducing locus of BALB/c mouse to chromosome 5. Science. 1979 Apr 6;204(4388):69–71. doi: 10.1126/science.219475. [DOI] [PubMed] [Google Scholar]
- Kozak C. A., Rowe W. P. Genetic mapping of the ecotropic virus-inducing locus Akv-2 of the AKR mouse. J Exp Med. 1980 Nov 1;152(5):1419–1423. doi: 10.1084/jem.152.5.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowy D. R., Chattopadhyay S. K., Teich N. M., Rowe W. P., Levine A. S. AKR murine leukemia virus genome: frequency of sequences in DNA of high-, low-, and non-virus-yielding mouse strains. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3555–3559. doi: 10.1073/pnas.71.9.3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majors J. E., Varmus H. E. Nucleotide sequences at host-proviral junctions for mouse mammary tumour virus. Nature. 1981 Jan 22;289(5795):253–258. doi: 10.1038/289253a0. [DOI] [PubMed] [Google Scholar]
- Meier H., Myers D. D., Huebner R. J. Genetic control by the hr-locus of susceptibility and resistance to leukemia. Proc Natl Acad Sci U S A. 1969 Jul;63(3):759–766. doi: 10.1073/pnas.63.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quint W., Quax W., van der Putten H., Berns A. Characterization of AKR murine leukemia virus sequences in AKR mouse substrains and structure of integrated recombinant genomes in tumor tissues. J Virol. 1981 Jul;39(1):1–10. doi: 10.1128/jvi.39.1.1-10.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rands E., Lowy D. R., Lander M. R., Chattopadhyay S. K. Restriction endonuclease mapping of ecotropic murine leukemia viral DNAs: size and sequence heterogeneity of the long terminal repeat. Virology. 1981 Jan 30;108(2):445–452. doi: 10.1016/0042-6822(81)90451-7. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rowe W. P., Hartley J. W., Bremner T. Genetic mapping of a murine leukemia virus-inducing locus of AKR mice. Science. 1972 Nov 24;178(4063):860–862. doi: 10.1126/science.178.4063.860. [DOI] [PubMed] [Google Scholar]
- Rowe W. P., Kozak C. A. Germ-line reinsertions of AKR murine leukemia virus genomes in Akv-1 congenic mice. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4871–4874. doi: 10.1073/pnas.77.8.4871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimotohno K., Mizutani S., Temin H. M. Sequence of retrovirus provirus resembles that of bacterial transposable elements. Nature. 1980 Jun 19;285(5766):550–554. doi: 10.1038/285550a0. [DOI] [PubMed] [Google Scholar]
- Staats J. Standardized nomenclature for inbred strains of mice: seventh listing for the International Committee on Standardized Genetic Nomenclature for Mice. Cancer Res. 1980 Jul;40(7):2083–2128. [PubMed] [Google Scholar]
- Steffen D. L., Bird S., Weinberg R. A. Evidence for the Asiatic origin of endogenous AKR-type murine leukemia proviruses. J Virol. 1980 Sep;35(3):824–835. doi: 10.1128/jvi.35.3.824-835.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen D. L., Taylor B. A., Weinberg R. A. Continuing germ line integration of AKV proviruses during the breeding of AKR mice and derivative recombinant inbred strains. J Virol. 1982 Apr;42(1):165–175. doi: 10.1128/jvi.42.1.165-175.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen D., Bird S., Rowe W. P., Weinberg R. A. Identification of DNA fragments carrying ecotropic proviruses of AKR mice. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4554–4558. doi: 10.1073/pnas.76.9.4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephension J. R., Reynolds R. K., Tronick S. R., Aaronson S. A. Distribution of three classes of endogenous type-C RNA viruses among inbred strains of mice. Virology. 1975 Oct;67(2):404–414. doi: 10.1016/0042-6822(75)90442-0. [DOI] [PubMed] [Google Scholar]
- Stephenson J. R., Aaronson S. A. Induction of an endogenous B-tropic type C RNA virus from SWR/J mouse embryo cells in tissue culture. Virology. 1976 Apr;70(2):352–359. doi: 10.1016/0042-6822(76)90277-4. [DOI] [PubMed] [Google Scholar]
- Traina V. L., Taylor B. A., Cohen J. C. Genetic mapping of endogenous mouse mammary tumor viruses: locus characterization, segregation, and chromosomal distribution. J Virol. 1981 Dec;40(3):735–744. doi: 10.1128/jvi.40.3.735-744.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Beveren C., Goddard J. G., Berns A., Verma I. M. Structure of Moloney murine leukemia viral DNA: nucleotide sequence of the 5' long terminal repeat and adjacent cellular sequences. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3307–3311. doi: 10.1073/pnas.77.6.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varmus H. E., Quintrell N., Ortiz S. Retroviruses as mutagens: insertion and excision of a nontransforming provirus alter expression of a resident transforming provirus. Cell. 1981 Jul;25(1):23–36. doi: 10.1016/0092-8674(81)90228-2. [DOI] [PubMed] [Google Scholar]