A large number of inhalation devices are used for delivery of asthma medication in children, and it is well known that the delivery device plays an essential role in lung dose of drug [1]. Therefore, there is a need for valid methods to estimate lung dose from different delivery devices to ensure evidence-based drug prescription in children. Recommendations cannot be based on surrogate measures of lung dose such as the fine particle mass, since these may not be valid measures of lung dose in vivo.
In adults it has been shown that early bioavailability of inhaled salbutamol can be used as an index of lung dose [2]. This is based on the fact that oropharyngeal and gastrointestinal absorption of salbutamol is negligible in the first 20 min after inhalation, and early systemic levels therefore reflect absorption from the lungs only [3]. However, the same may not be true for children, who often have faster absorption rates of drugs compared with adults [4].
We therefore performed a study to validate the 20-min pharmacokinetic profile as a measure of lung dose in children. The significance of oropharyngeal and early gastrointestinal absorption was investigated by actuating the pressurized metered-dose inhaler (pMDI) directly into the mouth after maximal inspiration to ensure oropharyngeal deposition only. The resultant serum profile after oropharyngeal deposition was then compared with the profile after inhalation measured in a previous study [5]. The two studies were similar regarding inclusion criteria, inhaled dose, study drug, delivery device, method of drug analysis and laboratory. The serum profiles between studies could therefore be compared.
Children aged 4–6 or 11–16 years, with a history of mild to moderate asthma in stable phase, were invited to participate in this study, which was approved by the Danish Medicines Agency (2612–1071) and the Ethics Committee of Copenhagen (KF. 02-079/99).
Rac-Salbutamol (Airomir; 3M Health Care, St Paul, MN, USA) was administered as an aerosol via a hydrofluoroalkanes pMDI with a non-electrostatic metal spacer attached [6]. The pMDI was actuated directly into the mouth after maximal inspiration to ensure oropharyngeal absorption. Children held their breath for as long as possible before exhaling. The pMDI was primed before inhalation, by firing four puffs into a plastic bag, and was shaken thoroughly immediately before each actuation. A total nominal dose of 400 µg salbutamol was administered as four separate doses of 0.1 mg. Blood samples were collected before and 10, 20, 30, 40 and 60 min after actuation of the pMDI. Blood sampling after 20 min (in contrast to the inhalation study) was performed to provide evidence that drug was actually deposited in the oropharynx and subsequently absorbed from the gastrointestinal tract after swallowing. Handling of blood samples and methods of serum analyses have been previously described [5]. The linear range for salbutamol detection in serum was 25.4–5070 pg ml−1.
Salbutamol concentrations were calculated as average (Cav) over 10–20 min. Mean Cav after oropharyngeal deposition and inhalation was compared in matched age-groups (4–6 years and 11–16 years, respectively). Mean values for groups were calculated as geometrics. Ratios of mean Cav after oropharyngeal deposition and inhalation were determined by Student's t-test of log-transformed values of Cav. A test not assuming variance homogeneity was appropriate for the young children, and an asymptotic 95% confidence interval (CI) was constructed.
Four patients aged 4–6 years and four patients aged 11–16 years participated. These were compared with 18 patients aged 4–6 years and 22 patients aged 11–16 years in the previous inhalation study. Four samples (11–16 years) with salbutamol levels below the linear range of quantification (25.4 pg ml−1) were given the value 25.4 pg ml−1.
The serum profile after oropharyngeal deposition showed a prolonged absorption phase in accordance with that seen after gastrointestinal absorption in adults [7] (Figure 1).
Figure 1.
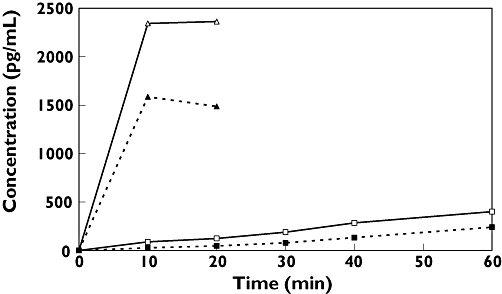
Salbutamol serum concentrations (geometric mean values) after oropharyngeal deposition or inhalation in different age groups (Inhalation 4–6 yr, (▵); Inhalation 11–16 yr, (▴); Oral deposition 4–6 yr, (□); Oral deposition 11–16 yr, (▪))
Cav after oropharyngeal deposition was 4.5% (95% CI 1.9, 10.7) of Cav after inhalation in children aged 4–6 years (absolute values 107 vs. 2360 pg ml−1) and 2.4% (95% CI 1.5, 3.9) in children aged 11–16 years (absolute values 38 vs. 1542 pg ml−1).
Early bioavailability after oropharyngeal deposition was <5% of bioavailability after inhalation for both younger and older children. This is probably an overestimation of the oropharyngeal contribution to bioavailability after inhalation, since our method includes the proportion of the emitted dose that is normally deposited in the spacer and the lungs.
Therefore, also in children the contribution of oropharyngeal and gastrointestinal absorption to early bioavailability after inhalation is minimal, and early bioavailability reflects absorption of drug deposited in the lungs. This means that the 20-min pharmacokinetic profile can be used to compare lung dose of delivery devices in children. We recommend that this method is used to test the performance of new salbutamol delivery devices instead of in vitro measures of lung dose, which may not be reliable measures of lung dose in vivo.
Competing interests
H.B. has been a consultant to, paid lecturer for and holds sponsored grants from Aerocrine, AstraZeneca, Altana, GSK, Merck and MedImmune. He does not hold stock or options in any pharmaceutical company in the respiratory field. K.B. has been a paid lecturer for and consultant to Merck. His ongoing PhD study is supported by unrestricted institutional grants from Aerocrine, AstraZeneca, GSK, Merck, and Medimmune.
Study medication and serum analyses were sponsored by 3M Pharma.
Supplementary material
The following supplementary material is available for this article online:
Individual salbutamol concentrations after oral deposition (60-min profile)
This material is available as part of the online article from http://www.blackwell-synergy.com
Please note: Blackwell Publishing is not responsible for the content or functionality of any supplementary materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
REFERENCES
- 1.Bisgaard H. Delivery of inhaled medication to children. J Asthma. 1997;34:443–67. doi: 10.3109/02770909709055389. [DOI] [PubMed] [Google Scholar]
- 2.Lipworth BJ. Pharmacokinetics of inhaled drugs. Br J Clin Pharmacol. 1996;42:697–705. doi: 10.1046/j.1365-2125.1996.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hindle M, Chrystyn H. Determination of the relative bioavailability of salbutamol to the lung following inhalation. Br J Clin Pharmacol. 1992;34:311–5. doi: 10.1111/j.1365-2125.1992.tb05921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rowland M, Tozer TN. Clinical Pharmacokinetics: Concepts and Applications. 3. Philadelphia, PA, London: Williams & Wilkins; 1995. [Google Scholar]
- 5.Bonnelykke K, Jespersen JJ, Bisgaard H. Age dependent systemic exposure to inhaled salbutamol. Br J Clin Pharmacol. 2007;64:241–4. doi: 10.1111/j.1365-2125.2007.02868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bisgaard H, Anhoj J, Klug B, Berg E. A non-electrostatic spacer for aerosol delivery. Arch Dis Child. 1995;73:226–30. doi: 10.1136/adc.73.3.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lipworth BJ, Clark RA, Dhillon DP, Moreland TA, Struthers AD, Clark GA, McDevitt DG. Pharmacokinetics, efficacy and adverse effects of sublingual salbutamol in patients with asthma. Eur J Clin Pharmacol. 1989;37:567–71. doi: 10.1007/BF00562546. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Individual salbutamol concentrations after oral deposition (60-min profile)
This material is available as part of the online article from http://www.blackwell-synergy.com
Please note: Blackwell Publishing is not responsible for the content or functionality of any supplementary materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.


