Abstract
The recently described nessy (Ncaph2nes/nes) mutant mouse strain has a defect in T-cell development caused by a mutation in the ubiquitous kleisin-β (also known as Ncaph2). Kleisin-β is a subunit of the condensin II complex involved in chromosome condensation during mitosis. The nessy phenotype is characterized by CD44hi CD8+ peripheral T cells, 10–20% of normal thymocyte numbers and 2·5-fold fewer αβ T cells in the spleen compared with wild-type mice. In this study we examined the effect of the nessy mutation in kleisin-β on the immune response by challenging mice with an attenuated strain of Salmonella. Results showed that nessy mice control bacterial load as effectively as wild-type mice but exhibit a reduced antibody titre. Further experiments revealed that while the T-dependent antibody response was diminished in nessy mice the T-independent response was normal, suggesting that the defect was the result of T-cell function and not B-cell function. In vitro activation assays showed that nessy T cells have a lower capacity to up-regulate the early activation marker CD69 than wild-type T cells. Upon transfer into RAG−/− mice, nessy and wild-type CD4 T cells showed equivalent homeostatic proliferation, while nessy CD8 T cells proliferated more than their wild-type counterparts. When cultured with anti-T-cell receptor β or concanavalin A, nessy T cells were found to die faster than wild-type T cells. These data indicate that kleisin-β is required for a normal immune response, and represent the first demonstration of a role for kleisin-β in T-cell function.
Keywords: kleisin, Ncaph2, Salmonella, T-cell activation, T-cell-dependent antibody
Introduction
The nessy mutant mouse strain was a product of an ethylnitrosourea (ENU) mutagenesis screen for immunological phenotypes.1,2 The strain was identified from the level of expression of the lymphocyte activation marker CD44 on CD8+ T cells. Normal mice have both CD44hi and CD44lo peripheral CD8+ T cells, but in nessy mice the CD44lo population is substantially reduced, leaving predominantly cells expressing high levels of CD44 on their surfaces. Further investigation into the nessy phenotype revealed a smaller than usual thymus, with only 10–20% of normal thymocyte numbers and an increase in the number of CD4− CD8− double-negative (DN) thymocytes with an accumulation at the CD25+ (DN2–3) subset.2 The number of T cells in the periphery is also reduced, albeit to a lesser extent than in the thymus, with approximately 2·5-fold fewer αβ T cells in the nessy spleen compared with the spleen of wild-type mice.2 The nessy mutant phenotype has been shown to be intrinsic to T cells by adoptive transfer experiments and no B-cell defects have been observed in nessy mice.2
The nessy defect is caused by a T to A substitution in the kleisin-β gene.2 In the mouse this gene has three potential splice variants differing slightly in the first exon. The nessy mutation results in an amino acid change of isoleucine to asparagine in the long form of the gene, while in the possible intermediate form it would lead to a serine to threonine change and would have no effect on the short form.2 Kleisin-β forms part of the ubiquitously expressed condensin II complex, which is involved in chromosome condensation during mitosis.3 The role of condensin II in T-cell function has not been examined previously.
The aim of this study was to examine the effect of the nessy kleisin-β mutation on the immune response, initially by challenging with a pathogen in vivo. Attenuated Salmonella strains are commonly used in mouse studies, and are ideal for this purpose as they elicit both CD44 and CD85 T-cell responses as well as an antibody response.6 Various mutant mice with immunological defects have proven unable to cope effectively when challenged with attenuated Salmonella strains. These include major histocompatibility complex class II-deficient (H-2I-Aβ−/−) mice and T-cell receptor β-deficient (TCR-β−/−) mice, which both suffered from more severe salmonellosis than wild-type mice,4 and CD28-deficient mice6,7 and mice lacking the interferon-γ receptor,4 which did not survive infection. On the other hand, B-cell-deficient μ−/− mice are able to control primary infection as efficiently as wild-type mice, although they exhibit delayed bacterial clearance upon reinfection with Salmonella.6 Given these findings, Salmonella infection was chosen as an ideal method to test the immune response of nessy mice.
In the present study we examined the immune response of the nessy mutant mouse to infection with an attenuated strain of Salmonella enterica serovar Dublin. Our results show that, while nessy mice were able to control bacterial load during infection as effectively as wild-type controls, they exhibited a diminished ability to produce antibody. To determine whether this was the result of defective T-cell help or of a B-cell-specific problem, and whether there was any evidence of skewing towards a T helper type 1 (Th1) or Th2 response, further immunization experiments were carried out using various T-cell-dependent and T-cell-independent antigens. The results indicated that both Th1 and Th2 T-cell-dependent antibody responses were diminished in the nessy mouse, while the T-independent response was unchanged. In vitro activation experiments were performed to compare the capacities of nessy and wild-type lymphocytes to become activated by stimulation through their antigen receptors. Results showed that nessy T cells had a lower capacity than their wild-type counterparts to up-regulate the early activation marker CD69. Homeostatic proliferation of nessy and wild-type T cells was also compared by transferring thymocytes into RAG−/− mice and allowing their expansion, then calculating the percentages of each cell type in the spleen. The in vivo proliferation of nessy CD4 thymocytes in this assay was equivalent to that of wild-type CD4 cells, while nessy CD8 T cells proliferated more than their wild-type counterparts. Finally, the rate of cell death in culture for nessy and wild-type T cells was measured and nessy cells were found to die faster than wild-type cells. Together these data suggest that the defect in nessy mice is associated with T cells rather than B cells, and that kleisin-β is required for a normal immune response. This is the first demonstration of a role for kleisin-β in T-cell function.
Materials and methods
Mice
The nessy mutant mouse strain was a product of the ENU mutagenesis project at the Australian Cancer Research Foundation (ACRF) laboratory, John Curtin School of Medical Research, Australian National University. All nessy, C57BL/6, B6Ly5a and RAG−/− mice used were bred at the ACRF. Both male and female mice were used; mice were matched for age and sex as closely as possible within each experiment. Ages ranged from 9 to 17 weeks at the time of infection/immunization.
Bacteria and inoculation
Salmonella enterica serovar Dublin strain SL56318 was used. Bacteria were grown in Luria–Bertani medium. Mice were inoculated by intraperitoneal injection with between 1·2 × 106 and 3·4 × 106 colony-forming units (CFU) from an overnight culture of bacteria as determined by optical density and using a growth curve. Bacteria were injected in phosphate-buffered saline (PBS).
Bacterial load determination
Bacterial load was measured in the livers and spleens of mice at given times postinfection (p.i.) by homogenizing organs and plating appropriate dilutions in PBS onto Luria–Bertani agar.
Immunization experiments
Mice were immunized intraperitoneally on day 0 with 50 μg chicken gammaglobulin (CGG; Jackson Immunoresearch Laboratories, West Grove, PA) and approximately 108 heat-killed Bordetella pertussis (BD Biosciences, Franklin Lakes, NJ), and were bled from the tail vein on day 14, or immunized on day 0 with 25 μg NP–Ficoll (4-hydroxy-3-nitrophenylacetyl aminoethylcarboxymethyl–Ficoll) (Biosearch Technologies, Novato, CA) and bled on day 7 postimmunization.
Enzyme-linked immunosorbent assay to detect antibodies
Anti-Salmonella antibodies [immunoglobulin M (IgM), IgG1, IgG2a and IgG3] were detected in plasma from blood taken at given times p.i. by enzyme-linked immunosorbent assay (ELISA). Ninety-six-well ELISA plates (Nunc, Roskilde, Denmark) were coated with SL5631 cell lysate using a protocol adapted from Valentine et al.9 Briefly, cells were lysed by sonication, protein concentration was determined, and approximately 12·5 μg protein was added to each well of a plate. For the immunization experiment, antibodies detected were IgG1, IgG2a and IgM specific to CGG, B. pertussis and NP–Ficoll, respectively. Plates were coated with 5 μg/ml CGG, 108/ml B. pertussis, or 20 μg/ml NP–bovine serum albumin (Biosearch Technologies). All plates were stored overnight at 4°, and washed with PBS + 0·05% Tween-20 before use and between steps. Antibody binding was blocked at the appropriate stages with PBS + 5% skim milk powder. Plasma was diluted 1/100 in PBS + 1% skim milk powder, and serially diluted across the plates using 1/2 log dilutions. Alkaline phosphatase-conjugated secondary antibodies (as listed above; Southern Biotech, Birmingham, AL) were used at 1/2000 in PBS + 1% skim milk powder, and detected using Sigma Fast p-nitrophenyl phosphate tablets (Sigma, St Louis, MO) dissolved in MilliQ water. Reactions were stopped using 50 μl 0·1 m ethylenediaminetetraacetic acid per well. Optical density (OD) was read at 405 nm and the inverse of plasma dilution was plotted against OD to determine antibody titre as the inverse of dilution at a given OD in the linear section of the curve. Minimum detectable titre was at a dilution of 1/100 (i.e. a titre of 100). For statistical analysis titres of less than 100 were designated as 100.
In vitro activation of lymphocytes
Splenocytes were cultured in RPMI-1640 supplemented with 10% fetal calf serum, 100 U/ml penicillin, 10 μg/ml streptomycin, 2 mm l-glutamine and 50 μm 2-mercaptoethanol. Cells were activated by plating on flat-bottomed 24-well or 96-well plates coated with anti-TCR for T-cell assays and with lipopolysaccharide (LPS) for B-cell assays, serially diluted across the plate. Anti-TCR was diluted in PBS and LPS was diluted in culture medium. Plates with anti-TCR were incubated overnight at 4° or for at least 2 hr at 37° and unbound antibody was removed before the addition of cells, while LPS added to the plates at the same time as the cells. Cells were cultured overnight then activation was measured by flow cytometry. Antibodies used included: phycoerythrin-conjugated anti-CD45.1, biotin-conjugated anti-CD45.2, fluorescein isothiocyanate-conjugated anti-CD69, phycoerythrin-Cy7-conjugated anti-CD4, allophycocyanin-Cy7-conjugated anti-CD8 and allophycocyanin-conjugated streptavidin (BD Biosciences).
In vivo homeostatic proliferation
Thymocytes were isolated from nessy and B6Ly5a mice and single cell suspensions were made. Cells were counted and a mixture of 5 × 106nessy and 5 × 106 B6Ly5a cells were injected intravenously into RAG−/− mice. Mice were killed 1, 2 or 4 weeks after cell transfer and splenocytes were analysed by flow cytometry.
Analysis of cell death in culture
Splenocytes from 10-week-old nessy (Ly5b) and B6Ly5a mice were cultured separately or together (at a ratio of 3 : 2) in 96-well plates at 5 × 106 cells/well in supplemented RPMI-1640 plus 1 μg/ml concanavalin A. Cells were collected after 0, 3, 20, 26, 45 or 70 hr in culture and analysed by flow cytometry with fluorescent-conjugated antibodies against CD45.1, CD45.2, CD4 and CD8 (as described above, BD Biosciences), plus 7-amino-actinomycin D (7-AAD) (eBioscience, San Diego, CA) to detect dead cells.
Statistical analysis
Significant differences were calculated using the two-tailed Student's t-test assuming unequal variance.
Results
Nessy mice control bacterial load as effectively as C57BL/6 mice
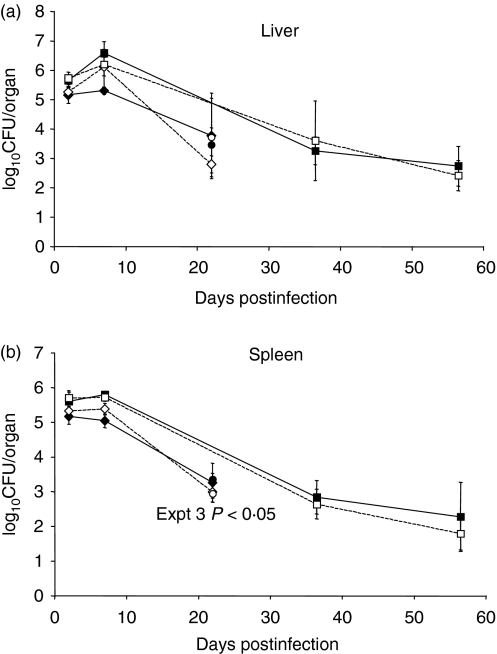
Nessy and control mice were inoculated with S. enterica serovar Dublin strain SL5631 and killed at certain times p.i., and bacterial load was determined for both the spleen and liver (Fig. 1). Significant differences were not found in the bacterial load in spleens and livers of nessy versus C57BL/6 mice at any of the time points in each of three experiments performed, except at day 22 p.i. in the third experiment. In the first experiment, nine nessy and nine C57BL/6 mice were inoculated with 1·6 × 106 CFU, and three mice from each strain were killed 2, 7 and 22 days p.i. In the second experiment 18 nessy and 18 C57BL/6 mice were inoculated with 3·4 × 106 CFU and three mice were killed at 2, 7, 36, 37, 56 and 57 days p.i. The results for days 36 and 37 have been combined, as have those for days 56 and 57. In the third experiment three nessy and three C57BL/6 mice were inoculated with 1·2 × 106 CFU and killed at 22 days p.i. The overall conclusion from the experiments measuring bacterial load was, therefore, that nessy mice were able to control infection by S. enterica serovar Dublin strain SL5631 as effectively as C57BL/6 mice at the doses employed.
Figure 1.
Bacterial load in liver and spleen of Salmonella-infected mice. Bacterial load [log10 colony-forming units (CFU)/organ] in (a) liver and (b) spleen of nessy and C57BL/6 mice killed at certain times after infection with Salmonella enterica serovar Dublin SL5631 in three separate experiments. Mice in experiment 1 received approximately 1·2 × 106 CFU and were killed at 2, 7 or 22 days postinfection (p.i.). Mice in experiment 2 received approximately 3·4 × 106 CFU and were killed at 2, 7, 36/37 or 56/57 days p.i. In experiment 3, mice received approximately 1·2 × 106 CFU and were killed at 22 days p.i. There is a significant difference between nessy and C57BL/6 bacterial loads from the spleen in experiment 3 (P < 0·05). Filled shapes represent nessy mice, open shapes represent C57BL/6 mice. Diamonds, experiment 1; squares, experiment 2; circles, experiment 3. Error bars represent standard deviation.
Nessy mice produce less anti-Salmonella antibody than C57BL/6 mice
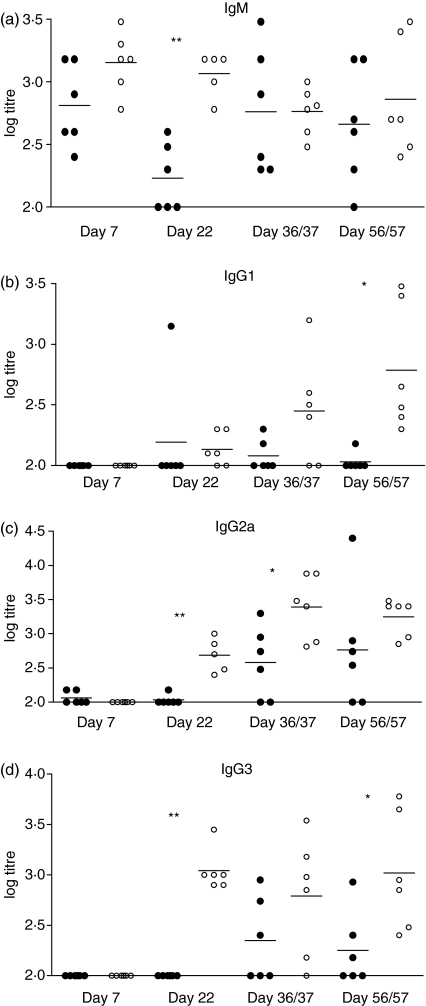
In addition to the T-cell response, antibody production is expected as a result of Salmonella infection. Production of Salmonella-specific IgM, IgG1, IgG2a and IgG3 antibodies in the plasma of blood from nessy and C57BL/6 mice were measured by ELISA. Titres of IgM were similar for nessy and control mice at all time-points with the exception of day 22 p.i. when nessy titres were significantly lower than wild-type titres (Fig. 2a). As expected, IgG1, IgG2a and IgG3 production were not detected at days 2 (data not shown) or 7 and were first detected at day 22. There was a trend towards lower production of IgG1 in nessy mice compared to wild-type mice, with the exception of an outlier on day 22 (Fig. 2b). A significant difference in IgG1 titres was recorded at day 56/57. IgG2a followed a similar trend of lower nessy titres (Fig. 2c) with significant differences at days 22 and 36/37, as did IgG3, which showed significant differences on days 22 and 56/57 (Fig. 2d). These results indicate a reduced ability of nessy mice to produce IgG antibodies in response to Salmonella infection.
Figure 2.
Nessy mice produce lower titres of anti-Salmonella antibodies. Titres of Salmonella enterica serovar Dublin-specific (a) immunoglobulin M (IgM), (b) IgG1, (c) IgG2a and (d) IgG3 from nessy (filled circles) and C57BL/6 (open circles) mice killed at the indicated times after infection. Plasma was obtained from blood taken at the time of death. Results represent titres from five or six mice at each time-point infected in three separate experiments. Data were analysed using a two-tailed Student’s t-test; significant differences are indicated above the corresponding data (*P < 0·05, **P < 0·005). Log 2·0 was the smallest measurable titre; titres at or below the detection limit were given a value of 2·0 for the calculation of means and statistical significance.
Nessy mice have reduced T-cell-dependent antibody production
While infection with Salmonella is a useful tool for studying the immune response due to the involvement of CD4, CD8 and B cells, Th1 cells are generally agreed to be the most important mediators of the acquired immune response to Salmonella as the bacteria enter the host macrophages, and Th1 cells can activate macrophages to destroy intracellular bacteria.4,10,11
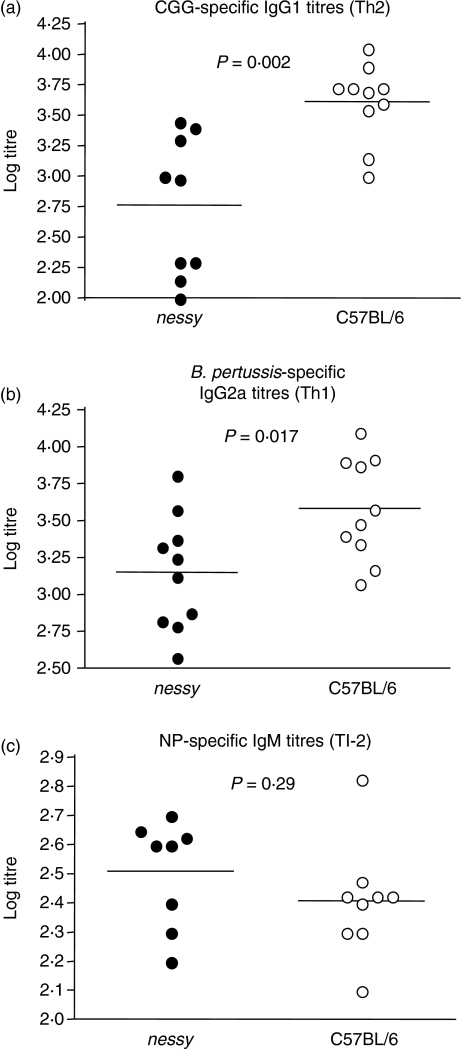
T-cell-dependent antibodies can also be classified as either Th1 or Th2 depending on whether B cells are stimulated by Th1 or Th2 cytokines.12 Both IgG2a, a Th1 antibody, and IgG1, a Th2 antibody, were measured and found to be reduced in Salmonella-infected nessy mice (Fig. 2). To examine the Th1 and Th2 responses more closely and to determine whether there was any skewing towards either response in nessy mice, a strategy was employed in which mice were immunized concurrently with the antigens CGG and killed B. pertussis, which elicit Th2 and Th1 T-cell-dependent antibody responses, respectively. Mice were immunized with 50 μg CGG and approximately 108 heat-killed B. pertussis, and were bled from the tail vein 14 days postimmunization. Levels of CGG-specific IgG1 and B. pertussis-specific IgG2a were measured by ELISA. Levels of both IgG1 and IgG2a were significantly reduced in nessy plasma compared to wild-type plasma (Fig. 3a,b). Therefore nessy mice showed no apparent skewing towards either a Th1 or a Th2 response but a reduction in both. This was consistent with the results of the Salmonella infection (Fig. 2).
Figure 3.
Nessy mice produce lower titres of helper-dependent immunoglobulin G1 (IgG1) and IgG2a antibodies. Nessy (closed circles) and C57BL/6 (open circles) mice were immunized with 50 μg chicken gamma globulin (CGG) and approximately 108 heat-killed Bordetella pertussis, and were bled from the tail vein 14 days postimmunization, or with 25 μg NP–Ficoll and bled from the tail vein 7 days postimmunization. Titres of IgG1 (a), IgG2a (b) or IgM (c) were determined by enzyme-linked immunosorbent assay. Data from three separate experiments are shown; averages are indicated by a horizontal bar. Data were analysed using Student's t-test; P values are indicated; data at or below the limit of detection (log titre of 2·0) are given a value of 2·0.
The nessy T-cell-independent antibody response is normal
The CGG/B. pertussis immunizations showed that there was clearly a reduction in T-cell-dependent antibody production in nessy mice. This could be the result of either a T-cell defect or a B-cell defect. The similarity in Salmonella-specific IgM titres for nessy and wild-type mice suggested that T-cell-independent antibody production was normal in nessy mice, and that it was therefore more likely to be a T-cell defect. The normal T-cell-independent response was confirmed by immunization of nessy and wild-type mice with NP–Ficoll, which elicited a T-cell-independent type II response. No significant difference between the nessy and wild-type IgM response to NP–Ficoll was observed (Fig. 3c).
These results strongly suggested that the nessy antibody production problem was related to the T cells rather than the B cells. The lower antibody production in nessy mice could be the result of the reduced T-cell number compared to wild-type mice,2 or of an intrinsic functional defect. The following experiments were designed to differentiate between these two possibilities.
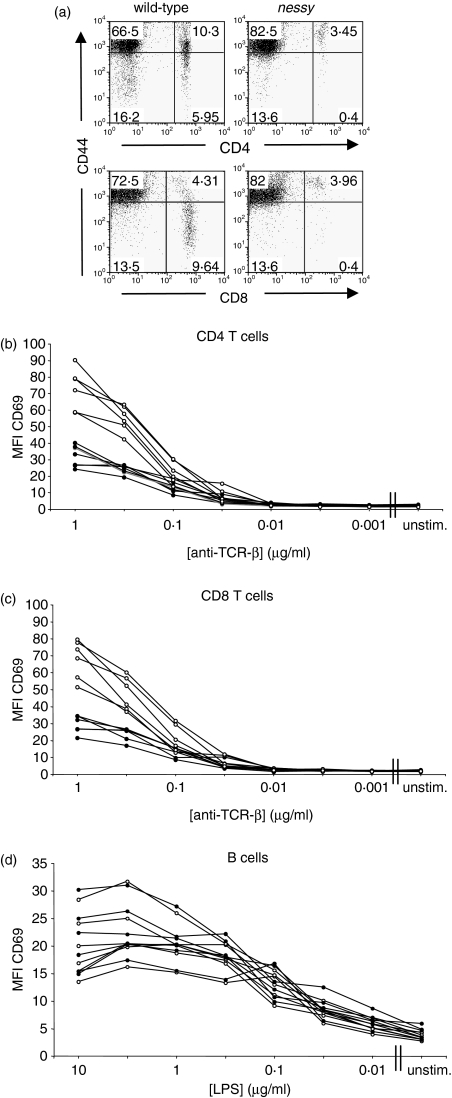
Nessy T cells have a reduced capacity for activation through the TCR
Figure 4(a) shows the typical CD44 expression on nessy and wild-type CD4 and CD8 T cells. The majority of nessy CD4 and CD8 T cells are CD44hi, while the wild-type cells include both CD44hi and CD44lo cells. The activated phenotype of nessy T cells supports the hypothesis of a possible T-cell defect. To analyse T-cell (and B-cell) function, the capacities of nessy and wild-type cells to become activated by stimulation through their antigen receptors were compared. This involved in vitro stimulation of splenocytes with either anti-TCR-β antibody to activate T cells or LPS to activate B cells, followed by analysis of the level of the early activation marker CD69 by flow cytometry. To minimize experimental variation and to eliminate potential differences caused by any trans-effects from other cell types in the culture, nessy (Ly5b) and wild-type (B6Ly5a) cells were cultured in the same wells. Representative results from one experiment plotting the mean fluorescence intensity of CD69 are shown in Fig. 4 (Fig. 4b, CD4 T cells; Fig. 4c, CD8 T cells and Fig. 4d, B cells). Both nessy and wild-type T cells up-regulated CD69 expression at a stimulus concentration of around 0·1 μg/ml anti-TCR-β, suggesting a similar threshold of activation. However, while CD69 levels on wild-type T cells continued to rise with increasing concentration of stimulus, the increase in expression of CD69 on nessy T cells was much less dramatic and the mean fluorescence intensity was about half that of wild-type at the highest stimulus concentration. In the case of B cells, nessy and wild-type cells up-regulated CD69 in a similar manner upon stimulation with LPS, which was once again consistent with the hypothesis that nessy B cells function normally.
Figure 4.
Nessy T cells up-regulate the activation marker CD69 to a lesser extent than wild-type cells. (a) Flow cytometry plots showing typical expression of CD44 on nessy and wild-type CD4 and CD8 T cells. Splenocytes from nessy and wild-type mice were analysed for expression of the cell surface markers CD4, CD8 and CD44 by flow cytometry. Plots on the left show cells from a wild-type mouse after gating on lymphocytes by size and granularity whereas those on the right show cells from a nessy mouse using the same gating. Note that the majority of nessy cells are CD44hi, while the wild-type cells include both CD44hi and CD44lo cells. (b–d) Nessy (Ly5b) (closed circles) and C57BL/6 (Ly5a) (open circles) splenocytes were cultured overnight together in a ratio of approximately 4 : 1. Cells were stimulated with anti-TCR-β or lipopolysaccharide (LPS) serially diluted in 1/2 log intervals. Expression of CD69 on CD4+ (b), CD8+ (c) and B (d) cells was measured by flow cytometry. Mean fluorescence intensity of CD69 is shown. Data shown are from one representative experiment from a total of three; unstim., unstimulated.
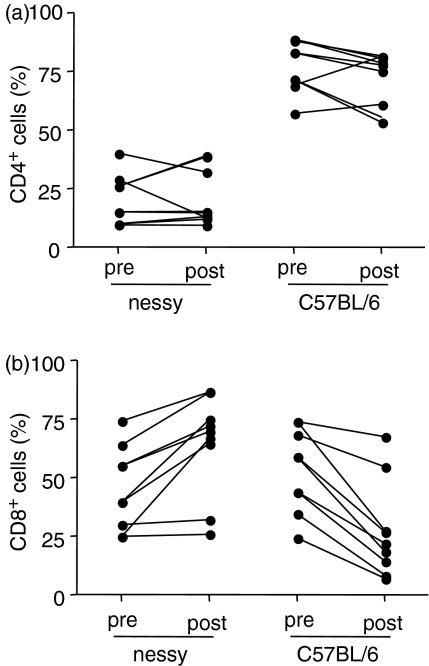
In vivo homeostatic proliferation of nessy T cells is equal to or more than that of wild-type cells
Thymocytes from nessy (Ly5ba) and wild-type (B6Ly5a) mice were cotransferred into RAG−/− mice by injection into the tail vein. By injecting thymocytes, only the mature single-positive cells will survive and proliferate. Double-negative and double-positive cells do not survive because they require the thymic environment to develop and are unable to reach the thymus from the blood stream.13 The cells were allowed to proliferate in the RAG−/− mice for approximately 2 weeks, at which time the mice were killed and their spleens were analysed by flow cytometry. Figure 5 shows the percentage of CD4+ T cells (upper graph) or CD8+ T cells (lower graph) that are nessy (Ly5b+) or wild-type (Ly5a+), before transfer (pre, i.e. thymocytes) and after expansion (post, i.e. splenocytes). The pre- and post-transfer data for individual mice are connected by a line. The results indicate that the relative percentages of nessy and wild-type CD4+ T cells changed very little while the relative percentage of nessy CD8+ T cells increased and that of wild-type CD8+ T cells decreased following expansion in RAG−/− mice.
Figure 5.
Comparison of percentages of CD4+ and CD8+ T cells that are nessy and wild-type before and after homeostatic expansion in RAG−/− hosts. Thymocytes were isolated from nessy (Ly5b) and C57BL/6 (Ly5a) mice and a mixture of 5 × 106nessy and 5 × 106 C57BL/6 cells were injected intravenously into RAG−/− mice. Mice were killed 2 weeks after cell transfer and splenocytes were labelled with antibodies against CD4, CD8, Ly5a and Ly5b and analysed by flow cytometry. The graphs show the percentage of CD4+ T cells (a) or CD8+ T cells (b) that are nessy (Ly5b+) or C57BL/6 (Ly5a+), before transfer (pre, i.e. thymocytes) and after expansion (post, i.e. splenocytes). The pretransfer values are for CD4+ and CD8+ single-positive thymocytes and do not include double-positives. Pretransfer and post-transfer values for individual mice are joined by a line. Data shown are combined from two experiments.
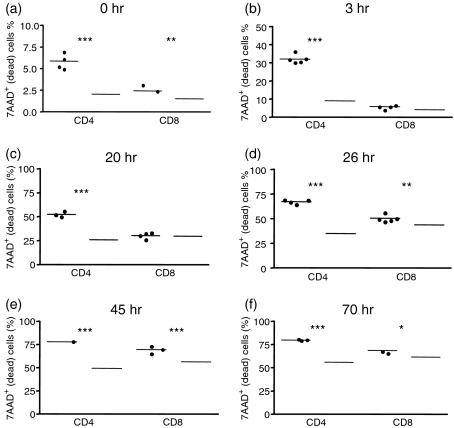
Nessy cells die faster than wild-type cells in culture
Cells were cultured with Concanavalin A over a period of 3 days and cell death was measured at various time-points by flow cytometry. Nessy (Ly5b) and B6Ly5a splenocytes were cultured separately or together and collected after 0, 3, 20, 26, 45 or 70 hr in culture. Cells were labelled with antibodies against Ly5a, Ly5b, CD4 and CD8, and stained with 7AAD to detect dead cells. The results from one of two such experiments are shown in Fig. 6. At all the time-points measured, nessy CD4+ T cells showed a significantly higher number of dead cells than wild-type CD4+ cells, while nessy CD8+ T cells showed similar levels of dead cells at the 0, 3 and 20 hr time-points but significantly more death than wild-type CD8+ cells at the 26, 45 and 70 hr time-points. Results were similar when nessy and wild-type cells were cultured either separately (data not shown) or together in the same well (Fig. 6).
Figure 6.
Nessy cells die faster in culture compared with wild-type cells. Nessy (Ly5b) (closed circles) and C57BL/6 (Ly5a) (open circles) splenocytes from 10-week-old mice mice were cultured separately (data not shown) or together (in a ratio of 3 : 2) in 96-well plates at 5 × 106 cells/well with 1 μg/ml Concanavalin A. Cells were collected after (a) 0, (b) 3, (c) 20, (d) 26, (e) 45 or (f) 70 hr in culture and analysed by flow cytometry. Cells were labelled with antibodies against Ly5a, Ly5b, CD4 and CD8, and stained with 7AAD to detect dead cells. Graphs show %7AAD+ (dead) cells after gating on lymphocytes by size and granularity, on Ly5a (C57BL/6) or Ly5b (nessy) and then on CD4 or CD8. Data shown are from one of two experiments in which similar trends were observed. P values are indicated (* < 0·05, ** < 0·01, *** < 0·005).
Discussion
The data presented in this study indicate that nessy mice have a T-cell functional defect in addition to the developmental defect previously described.2Nessy mice are able to control bacterial infection effectively, but show a reduction in antibody production. Immunization with various antigens has demonstrated that T-cell-dependent, but not T-cell-independent, antibody production is perturbed in nessy mice. There is no evidence of skewing towards a Th1 or Th2 response, rather both Th1 and Th2 responses are decreased. These data suggest that nessy B cells function normally while nessy T cells are defective.
To examine the immune response, nessy and wild-type mice were infected with an attenuated strain of Salmonella. The nessy mice were able to cope effectively with infection, showing little difference in bacterial load from the wild-type controls (Fig. 1). The most striking difference observed was a reduction in antibody production by nessy mice (Fig. 2). This could be the result of a T-cell functional defect, as antibody production involves T-cell help to stimulate B cells to start making antibody. However, it could also indicate a problem with B cells, either in their ability to respond to T-cell help or to make antibody.
This led to a more in-depth look at antibody production with the aim of determining whether the problem was the result of a defect of T or B cells. Mice were immunized with antigens to induce either T-cell-dependent or T-cell-independent antibody responses. The CGG was used to induce a Th2-type antibody response with the production of IgG1, killed B. pertussis was used to induce a Th1-type response with production of IgG2a, and NP–Ficoll was used to produce a T-cell-independent response with production of IgM. Results indicated that the Th1 and Th2 antibody responses were significantly reduced for nessy mice, while the T-cell-independent response was normal (Fig. 3). This suggested that there was a T-cell-related defect affecting antibody production (particularly the later antibody response or isotype switching) in nessy mice, and that B-cell function was most probably normal.
One possibility is that the T-cell-related problem was simply one of reduced cell numbers, as there are approximately 2·5-fold fewer peripheral T cells in the nessy mouse compared to wild-type C57BL/6.2 To determine whether or not nessy T cells have an intrinsic functional defect, in vitro assays for T-cell function were performed, including an activation assay examining expression of the early activation marker CD69. The results of the in vitro activation assay showed that nessy T cells started to up-regulate the early activation marker CD69 at the same threshold of stimulus concentration as wild-type cells (Fig. 4a). This result is inconsistent with published data indicating that cells with a CD44hi memory phenotype have a lower threshold for activation than naive cells.14 However, the published study used stimulation with antigen rather than anti-TCR stimulation, which may account for the difference. Although the threshold for stimulation was the same for nessy and wild-type cells, the nessy cells did not reach the same level of expression (mean fluorescence intensity) with higher concentrations of stimulus.
Nessy T cells did not demonstrate a proliferative disadvantage upon thymocyte transfer to RAG−/− mice, in fact nessy CD8+ T cells apparently proliferate more than their wild-type counterparts (Fig. 5). One possibility is that the initial CD44hi phenotype of the transferred nessy single-positive thymocytes may give the cells a temporary memory-like proliferative advantage, consistent with the results of Veiga-Fernandes et al.15 and Sprent et al.16 who found that CD8+ T cells with a CD44hi phenotype showed a faster rate of proliferation in vivo than naive cells. However, Neujahr et al.17 found that CD4+ memory cells also begin to proliferate faster than naive cells when transferred into a lymphopenic host, which is not consistent with the data showing that nessy CD4+ CD44hi cells proliferate at an equivalent rate to naive wild-type cells when transferred into RAG−/− mice. Alternatively, the difference in CD8 T-cell proliferation may be intrinsic to the nessy phenotype but unrelated to CD44 expression.
Finally, the results of the assay measuring cell death in vitro showed that nessy T cells die at a faster rate than their wild-type counterparts when cultured with Concanavalin A. The nessy CD4+ T cells in particular showed a significantly higher percentage of dead cells compared with wild-type CD4+ cells at all the time-points measured (0, 3, 20, 26, 45 and 70 hr), while significantly more nessy CD8+ T cells were dead compared with wild-type CD8+ cells at the later time-points of 26, 45 and 70 hr in culture (Fig. 6). One possible reason for nessy T cells to die faster in vitro is their CD44hi phenotype. CD44 has been shown to be involved in activation-induced cell death (AICD),18,19 which is consistent with the nessy cells dying faster than wild-type cells. The difference between CD4+ and CD8+ cell death could be the result of a difference in the inherent susceptibility of cells to AICD. This has been observed in various strains of mice, for example in non-obese diabetic (NOD) mice CD4+ T cells are more susceptible to AICD than CD8+ T cells, while the opposite is true in the non-obese resistant (NOR) mouse strain.20
One question raised by these observations is how the T-cell defect in nessy mice leads to reduced IgG antibody production. Isotype switching from IgM to IgG requires help from activated T cells, in particular CD4+ T cells.21 We have observed that both CD4 and CD8 T cells from the nessy mouse display defective T-cell activation. Being less activated than normal T cells, it is likely that nessy T cells have a reduced ability to help B cells to switch from IgM to IgG. This would explain the reduced levels of class-switched antibodies produced in nessy mice in response to stimulation with T-cell-dependent antigens while production of T-cell-independent antibodies is unaffected.
Another intriguing question is why a defect in kleisin-β affects CD4 and CD8 T cells differently. To this end, it is important to note that this mutation in a ubiquitous protein only affects one cell type. Our hypothesis is that kleisin-β is most likely acting through an epigenetic mechanism. Epigenetic regulators have different effects in different cell types, for example inactivating different regions of DNA.22 Epigenetic regulation is critical during cell differentiation, allowing cells to remember cell fate decisions, and has been shown to be important for T-cell development.23 For example, Brg-1, a catalytic subunit of mammalian chromatin remodelling complexes, regulates the expression of CD8 through the suppression of CD4,24,25 while the chromatin remodeller Mi2β is required for the expression of CD4 through association with the CD4 enhancer.26 Epigenetic regulation is also important in the control of T-cell function, with the chromatin remodelling of the Ifng and Il4 genes controlling the differentiation of naive T cells into Th1 or Th2 cells.27 We hypothesize that the differences in epigenetic regulation of CD4 and CD8 T cells are small enough that both are affected by the nessy mutation to the exclusion of all other cells types but large enough to allow differential effects of the mutation to be observed.
Emerging evidence of a role for condensins in transcriptional regulation in other organisms and cell types supports the hypothesis that epigenetic control of T-cell gene expression is altered by the nessy mutation. In Caenorhabditis elegans, transcription of X-linked genes is globally tuned down by a factor of two in XX individuals through a process that depends upon the condensin components, including the C. elegans kleisin dpy-26.28 Interaction with a SET-domain histone methyltransferase and a zinc finger protein appears to recruit the condensins to loading regions along the X chromosome, from which they become concentrated at sites of transcription initiation at densities correlated with the rate of transcription. Interestingly, a condensin kleisin mutation in Drosophila, barren, results in abnormal epigenetic silencing of transcription across adjacent body segments that resembles mutations in polycomb SET domain proteins.29 In yeast, mutations in condensin subunits, including kleisin, prevent the normal transcriptional silencing of the ribosomal RNA gene cluster during the nutrient starvation of treatment with rapamycin.30 The condensin complexes are recruited to the rDNA cluster during these responses by a process that requires specific histone deacetylases, and the condensins are required to initiate and maintain condensation of this specific locus. In mammalian cells, the heat-shock protein gene hsp 70i is spared from undergoing chromosomal condensation during the M phase of the cell cycle, and this is achieved by binding interactions between a specific DNA binding protein for the locus, HSF2, Protein phosphatase 2a, and the condensin subunit CAP-G.31 The interaction promotes dephosphorylation of CAP-G and CAP-H/kleisin, so opposing the effects of M-phase cyclin kinase, and enabling the locus to be rapidly expressed in response to stimuli at the start of the G1 phase. The mammalian CAP-G2 condensin subunit is recruited by the specific DNA-binding transcription factors SCL and E12 to repress specific gene expression and promote differentiation in erythrocyte precursors.32
Taken together, these recent results suggest that targeting and loading of condensins on specific regions and genes – through interactions with specific DNA binding proteins and general chromatin remodelling complexes – promote varying degrees of chromatin condensation to regulate gene transcription. It is thus plausible that the nessy point mutation diminishes the targeting and loading of condensins to specific loci in T cells, causing overexpression of particular genes. Future studies using chromatin immunoprecipitation will be necessary to test this hypothesis.
This study follows on from our previous work showing a completely unexpected, T-cell-specific effect of a point mutation in kleisin-β2 Kleisin-β is a component of the ubiquitous condensin II complex that has a known role in chromosome condensation. Condensin II has been shown to be important for mitosis and has also been implicated in epigenetic regulation of gene expression. This study represents the first demonstration of a role for kleisin-β in T-cell function, providing a possible link between T-cell biology and chromosome structure. It remains to be determined whether the nessy T-cell defect is caused by disruption of the chromosome condensation function of kleisin-β (e.g. through an epigenetic effect), or whether kleisin-β has an entirely novel function in T cells. In either case, the characterization of the immune function of the nessy mouse, revealing the novel role of kleisin-β in T-cell function, provides an important contribution to our understanding of the immune system.
Acknowledgments
This work was supported by grants from the National Health and Medical Research Council, and the Australian Capital Territory Cancer Council. The authors wish to thank Dr Carola Vinuesa for helpful advice, and members of the Verma, Fahrer and Goodnow Laboratories for constructive discussions.
References
- 1.Nelms KA, Goodnow CC. Genome-wide ENU mutagenesis to reveal immune regulators. Immunity. 2001;15:409–18. doi: 10.1016/s1074-7613(01)00199-6. [DOI] [PubMed] [Google Scholar]
- 2.Gosling KM, Makaroff LE, Theodoratos A, et al. A mutation in a chromosome condensin II subunit, kleisin-beta, specifically disrupts T cell development. Proc Natl Acad Sci USA. 2007;104:12445–50. doi: 10.1073/pnas.0704870104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schleiffer A, Kaitna S, Maurer-Stroh S, Glotzer M, Nasmyth K, Eisenhaber F. Kleisins: a superfamily of bacterial and eukaryotic SMC protein partners. Mol Cell. 2003;11:571–5. doi: 10.1016/s1097-2765(03)00108-4. [DOI] [PubMed] [Google Scholar]
- 4.Hess J, Ladel C, Miko D, Kaufmann SHE. Salmonella typhimurium aroA- infection in gene-targeted immunodeficient mice. J Immunol. 1996;156:3321–6. [PubMed] [Google Scholar]
- 5.Yrlid U, Wick MJ. Salmonella-induced apoptosis of infected macrophages results in presentation of a bacteria-encoded antigen after uptake by bystander dendritic cells. J Exp Med. 2000;191:613–23. doi: 10.1084/jem.191.4.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McSorley SJ, Jenkins MK. Antibody is required for protection against virulent but not attenuated Salmonella enterica serovar Typhimurium. Infect Immun. 2000;68:3344–8. doi: 10.1128/iai.68.6.3344-3348.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mittrucker H-W, Kohler A, Mak TK, Kaufmann SHE. Critical role of CD28 in protective immunity against Salmonella typhimurium. J Immunol. 1999;163:6769–76. [PubMed] [Google Scholar]
- 8.Segall T, Lindberg AA. Salmonella dublin experimental infection in calves: protection after oral immunization with an auxotrophic aroA live vaccine. Zentralbl Veterinarmed. 1991;38:142–60. doi: 10.1111/j.1439-0450.1991.tb00857.x. [DOI] [PubMed] [Google Scholar]
- 9.Valentine PJ, Devore BP, Heffron F. Identification of three highly attenuated Salmonella typhimurium mutants that are more immunogenic and protective in mice than a prototypical aroA mutant. Infect Immun. 1998;66:3378–83. doi: 10.1128/iai.66.7.3378-3383.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mittrucker H-W, Kaufmann SHE. Immune response to infection with Salmonella typhimurium in mice. J Leukoc Biol. 2000;67:457–63. doi: 10.1002/jlb.67.4.457. [DOI] [PubMed] [Google Scholar]
- 11.Mittrucker H-W, Kohler A, Kaufmann SHE. Characterization of the murine T-lymphocyte response to Salmonella enterica serovar Typhimurium infection. Infect Immun. 2002;70:199–203. doi: 10.1128/IAI.70.1.199-203.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abbas AK, Burstein HJ, Bogen SA. Determinants of helper T cell-dependent antibody production. Semin Immunol. 1993;5:441–7. doi: 10.1006/smim.1993.1050. [DOI] [PubMed] [Google Scholar]
- 13.Goldschneider I, Komschlies KL, Greiner DL. Studies of thymocytopoiesis in rats and mice. I. Kinetics of appearance of thymocytes using a direct intrathymic adoptive transfer assay for thymocyte precursors. J Exp Med. 1986;163:1–17. doi: 10.1084/jem.163.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Curtsinger JM, Lins DC, Mescher MF. CD8+ memory T cells (CD44high, Ly-6C+) are more sensitive than naive cells to (CD44low, Ly-6C−) to TCR/CD8 signaling in response to antigen. J Immunol. 1998;160:3236–43. [PubMed] [Google Scholar]
- 15.Veiga-Fernandes H, Walter U, Bourgeois C, McLean A, Rocha B. Response of naive and memory CD8+ T cells to antigen stimulation in vivo. Nat Immunol. 2000;1:47–53. doi: 10.1038/76907. [DOI] [PubMed] [Google Scholar]
- 16.Sprent J, Zhang X, Sun S, Tough D. T-cell proliferation in vivo and the role of cytokines. Philos Trans R Soc Lond B Biol Sci. 2000;355:317–22. doi: 10.1098/rstb.2000.0568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neujahr DC, Chen C, Huang X, et al. Accelerated memory cell homeostasis during T cell depletion and approaches to overcome it. J Immunol. 2006;176:4632–9. doi: 10.4049/jimmunol.176.8.4632. [DOI] [PubMed] [Google Scholar]
- 18.Foger N, Marhaba R, Zoller M. CD44 supports T cell proliferation and apoptosis by apposition of protein kinases. Eur J Immunol. 2000;30:2888–99. doi: 10.1002/1521-4141(200010)30:10<2888::AID-IMMU2888>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 19.Nakano K, Saito K, Mine S, Matsushita S, Tanaka Y. Engagement of CD44 up-regulates Fas Ligand expression on T cells leading to activation-induced cell death. Apoptosis. 2007;12:45–54. doi: 10.1007/s10495-006-0488-8. [DOI] [PubMed] [Google Scholar]
- 20.Arreaza G, Salojin K, Yang W, et al. Deficient activation and resistance to activation-induced apoptosis of CD8+ T cells is associated with defective peripheral tolerance in nonobese diabetic mice. Clin Immunol. 2003;107:103–15. doi: 10.1016/s1521-6616(03)00049-4. [DOI] [PubMed] [Google Scholar]
- 21.Mills DM, Cambier JC. B lymphocyte activation during cognate interactions with CD4+ T lymphocytes: molecular dynamics and immunologic consequences. Semin Immunol. 2003;15:325–9. doi: 10.1016/j.smim.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 22.Wilson CB, Makar KW, Shnyreva M, Fitzpatrick DR. DNA methylation and the expanding epigenetics of T cell lineage commitment. Semin Immunol. 2005;17:105–19. doi: 10.1016/j.smim.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 23.Wilson CB, Merkenschlager M. Chromatin structure and gene regulation in T cell development and function. Curr Opin Immunol. 2006;18:143–51. doi: 10.1016/j.coi.2006.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chi TH, Wan M, Lee PP, Akashi K, Metzger D, Chambon P, Wilson CB, Crabtree GR. Sequential roles of Brg, the ATPase subunit of BAF chromatin remodeling complexes, in thymocyte development. Immunity. 2003;19:169–82. doi: 10.1016/s1074-7613(03)00199-7. [DOI] [PubMed] [Google Scholar]
- 25.Gebuhr TC, Kovalev GI, Bultman S, Godfrey V, Su L, Magnuson T. The role of Brg1, a catalytic subunit of mammalian chromatin-remodeling complexes, in T cell development. J Exp Med. 2003;198:1937–49. doi: 10.1084/jem.20030714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams CJ, Naito T, Arco PG, et al. The chromatin remodeler Mi-2beta is required for CD4 expression and T cell development. Immunity. 2004;20:719–33. doi: 10.1016/j.immuni.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 27.Reiner SL. Epigenetic control in the immune response. Hum Mol Genet. 2005;1:R41–6. doi: 10.1093/hmg/ddi115. [DOI] [PubMed] [Google Scholar]
- 28.Ercan S, Giresi PG, Whittle CM, Zhang X, Green RD, Lieb JD. X chromosome repression by localization of the C. elegans dosage compensation machinery to sites of transcription initiation. Nat Genet. 2007;39:403–8. doi: 10.1038/ng1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lupo R, Breiling A, Bianchi ME, Orlando V. Drosophila chromosome condensation proteins Topoisomerase II and Barren colocalize with Polycomb and maintain Fab-7 PRE silencing. Mol Cell. 2001;7:127–36. doi: 10.1016/s1097-2765(01)00161-7. [DOI] [PubMed] [Google Scholar]
- 30.Tsang CK, Li H, Zheng XS. Nutrient starvation promotes condensin loading to maintain rDNA stability. EMBO J. 2007;26:448–58. doi: 10.1038/sj.emboj.7601488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xing H, Wilkerson DC, Mayhew CN, et al. Mechanism of hsp70i gene bookmarking. Science. 2005;307:421–3. doi: 10.1126/science.1106478. [DOI] [PubMed] [Google Scholar]
- 32.Xu Y, Leung CG, Lee DC, Kennedy BK, Crispino JD. MTB, the murine homolog of condensin II subunit CAP-G2, represses transcription and promotes erythroid cell differentiation. Leukemia. 2006;20:1261–9. doi: 10.1038/sj.leu.2404252. [DOI] [PubMed] [Google Scholar]