Abstract
The endogenous cannabinoid system plays an important role in regulating the immune system. Modulation of endogenous cannabinoids represents an attractive alternative for the treatment of inflammatory disorders. This study investigated the effects of URB597, a selective inhibitor of fatty acid amide hydrolase (FAAH), the enzyme catalysing degradation of the endogenous cannabinoid anandamide, and AM404, an inhibitor of anandamide transport, on lipopolysaccharide (LPS)-induced increases in plasma cytokine levels in rats. Both URB597 and AM404 potentiated the LPS-induced increase in plasma tumour necrosis factor-α (TNF-α) levels. The peroxisome proliferator-activated receptor γ (PPARγ) antagonist, GW9662, attenuated the AM404-induced augmentation of TNF-α levels. Furthermore, the selective cannabinoid CB1 and CB2 receptor antagonists, AM251 and AM630 respectively, and the transient receptor potential vanilloid receptor-1 (TRPV1) antagonist, SB366791, reduced LPS-induced TNF-α plasma levels both alone and in combination with AM404. In contrast, AM404 inhibited LPS-induced increases in circulating interleukin-1β (IL-1β) and IL-6. AM251 attenuated the immunosuppressive effect of AM404 on IL-1β. None of the antagonists altered the effect of AM404 on LPS-induced IL-6. Moreover, AM251, AM630 and SB366791, administered alone, inhibited LPS-induced increases in plasma IL-1β and IL-6 levels. In conclusion, inhibition of endocannabinoid degradation or transport in vivo potentiates LPS-induced increases in circulating TNF-α levels, an effect which may be mediated by PPARγ and is also reduced by pharmacological blockade of CB1, CB2 and TRPV1. The immunosuppressive effect of AM404 on IL-1β levels is mediated by the cannabinoid CB1 receptor. Improved understanding of endocannabinoid-mediated regulation of immune function has fundamental physiological and potential therapeutic significance.
Keywords: anandamide, interleukin-1β, interleukin-6, rodent, tumour necrosis factor-α
Introduction
The endocannabinoid system comprises two known cannabinoid receptor subtypes, CB1 and CB2,1,2 a number of endogenous ligands (endocannabinoids) including the compounds anandamide and 2-arachidonoyl glycerol (2-AG),3 a high affinity reuptake transport system4,5 and endocannabinoid synthesizing and metabolizing enzymes.6,7 Endocannabinoid actions are terminated by removal from the extracellular space, for anandamide by the anandamide membrane transporter, and subsequent enzymatic degradation. The enzyme fatty acid amide hydrolase (FAAH) preferentially metabolizes anandamide6 and although 2-AG also acts as a substrate for FAAH, monoglyceride lipase is considered the primary enzyme involved in 2-AG inactivation.7 In addition to CB1 and CB2 receptors, endocannabinoids also have affinity for transient receptor potential vanilloid 1 (TRPV1), peroxisome proliferator-activated receptors (PPARs) and the orphan receptor GPR55.8–10
There is increasing evidence to suggest that cannabinoids modulate the immune system and represent an important target for the treatment of inflammatory disorders. Studies in vitro have demonstrated that anandamide attenuates lipopolysaccharide (LPS) -induced increases in both protein and messenger RNA levels of proinflammatory cytokines [tumour necrosis factor-α (TNF-α), interleukin-1β (IL-1β) and IL-6] from macrophages and microglial cells11–13 and inhibits lymphocyte proliferation.14 In addition, anandamide has also been reported to increase proinflammatory cytokines under certain conditions.15 However, there is a paucity of data regarding the immunological effects of enhancing endocannabinoid tone in vivo. The present study tested the hypothesis that inhibition of FAAH or blockade of anandamide transport modulates LPS-induced increases in circulating levels of the proinflammatory cytokines TNF-α, IL-1β and IL-6. URB597 is a selective inhibitor of FAAH that does not interact with other cannabinoid-related targets including receptors, transport systems or enzymes.16–18 Anti-inflammatory effects of URB597 have been demonstrated both in animal models19,20 and in microglial cell cultures.21 AM404 is an inhibitor of endocannabinoid transport, known to enhance extracellular anandamide levels.4,22In vitro studies have shown that inhibition of anandamide transport reduces the production of the proinflammatory cytokines IL-1β and IL-12 from macrophages.23
Our work and that of others has previously demonstrated that the selective CB1 and CB2 receptor antagonists, SR141716A (rimonabant) and SR144528 respectively, attenuate LPS-induced increases in proinflammatory cytokines in a manner similar to that observed for cannabinoid receptor agonists.24–26 To explore if immunosuppression results from administration of other cannabinoid receptor antagonists, the effects of the selective CB1 and CB2 receptor antagonists, AM251 and AM630 respectively, were investigated in the present study. Their inclusion also allowed the investigation of the receptor subtype through which anandamide may mediate its effects on LPS-induced plasma cytokine levels. As discussed above, increasing evidence suggests that endocannabinoids may mediate their effects via alternative, non-CB1/CB2 receptor targets. In light of this, the effects of the TRPV1 and PPARγ receptor antagonists, SB366791 and GW9662 respectively, on LPS-induced cytokine levels, alone and in combination with AM404, were examined.
Materials and methods
Animals
Experiments were carried out on male Sprague Dawley rats (weight 220–260 g; Harlan, Bicester, UK), housed singly in plastic-bottomed cages (45 × 25 × 20 cm) containing wood shavings as bedding. The animals were maintained in a constant temperature (21 ± 2°) under standard lighting conditions (12 : 12 hr light–dark, lights on from 08.00 to 20.00 hr). All experiments were carried out during the light phase, between 08.30 and 15.00 hr. Food and water were available ad libitum. Animals were habituated to handling and received an intraperitoneal (i.p.) injection of sterile saline (0·89% NaCl) for 3–4 days before the experimentat to minimize the influence of the injection procedure on biological endpoints. The experimental protocol was carried out in accordance with the guidelines of the Animal Welfare Committee, National University of Ireland, Galway under licence from the Irish Department of Health and Children and in compliance with the European Communities Council directive 86/609.
Experimental design
Experiment 1: The effect of URB597, AM251 and AM630 alone and in combination on LPS-induced cytokine levels in plasma. Rats were randomly assigned to one of seven groups: Vehicle–Vehicle + Saline (n = 7); Vehicle–Vehicle + LPS (n = 7); AM251–Vehicle + LPS (n = 9); AM630–Vehicle + LPS (n = 9); Vehicle–URB597 + LPS (n = 9); AM251–URB597 + LPS (n = 7); AM630–URB597 + LPS (n = 6).
Rats were injected with the CB1 and CB2 receptor antagonists, AM251 (3 mg/kg i.p.) and AM630 (3 mg/kg i.p.), respectively, or vehicle (ethanol : cremophor : saline; 1 : 1 : 18) at an injection volume of 2 ml/kg, followed immediately by an injection of URB597 (0·6 mg/kg i.p.) or vehicle (ethanol : cremophor : saline; 1 : 1 : 18). The dose of the FAAH inhibitor URB597 was selected based on studies demonstrating anti-inflammatory effects at a 50% effective dose of approximately 0·3 mg/kg.19 The time of URB597 administration was determined based on the finding that inhibition of FAAH peaks 1 hr after i.p. injection and decreases thereafter.16 Antagonist doses were chosen based on previous studies demonstrating their ability to block the effects of cannabinoid agonists in vivo.27,28 Rats were injected with the cannabinoids 30 min before an i.p. injection of either LPS (100 μg/kg) or saline vehicle (sterile 0·89% NaCl) administered at a volume of 1 ml/kg. The dose and time of LPS administration were chosen on the basis of previous work in our laboratory demonstrating increases in peripheral cytokine (TNF-α, IL-1β, IL-6) levels 2 hr after LPS administration.26,29 Blood samples were taken 2 hr after administration of LPS or saline, via cardiac puncture into a heparinized syringe under CO2 anaesthesia. Blood samples were centrifuged at 14 000 g for 15 min at 4° to obtain plasma, which was removed and stored at −80° until cytokine determination.
Experiment 2(a): The effect of AM404 on LPS-induced cytokine levels in plasma. Rats were randomly assigned to one of six experimental groups: Vehicle + Saline (n = 9), AM404 (20 mg/kg) + Saline (n = 8), Vehicle + LPS (n = 9), AM404 (5 mg/kg) + LPS (n = 8), AM404 (10 mg/kg) (n = 8) + LPS and AM404 (20 mg/kg) + LPS (n = 7).
Rats received either the anandamide transport inhibitor AM404 (5, 10, 20 mg/kg i.p.) or vehicle (ethanol : cremophor : saline; 1 : 1 : 18) at an injection volume of 2 ml/kg. The time and doses of AM404 administration were based on previous published studies demonstrating increased plasma levels of anandamide following AM404 administration4,22 and inhibitory effects on proinflammatory cytokine levels.30 LPS (100 μg/kg) or saline vehicle (sterile 0·89% NaCl) was administered 1 hr following AM404 or vehicle injection at a volume of 1 ml/kg. Blood samples were taken 2 hr after administration of LPS or saline; plasma was obtained as previously described and stored at −80° until cytokine determination.
Experiment 2(b): The effect of cannabinoid receptor, vanilloid receptor and PPARγ antagonists on AM404-induced changes in plasma cytokine levels following LPS administration. Rats were randomly assigned to one of nine groups: Vehicle–Vehicle + Saline (n = 11); Vehicle–Vehicle + LPS (n = 9); SB366791–Vehicle + LPS (n = 8); GW9662–Vehicle + LPS (n = 7), Vehicle–AM404 + LPS (n = 10); AM251–AM404 + LPS (n = 10); AM630–AM404 + LPS (n = 9); SB366791–AM404 + LPS (n = 8); GW9662–AM404 + LPS (n = 8).
Rats were injected with the CB1 receptor antagonist AM251 (3 mg/kg i.p.), the CB2 receptor antagonist AM630 (3 mg/kg i.p.), the TRPV1 antagonist SB366791 (2 mg/kg), the PPARγ antagonist GW9662 (2 mg/kg) or vehicle (ethanol : cremophor : saline; 1 : 1 : 18) at an injection volume of 2 ml/kg, followed immediately by an i.p. injection of AM404 (20 mg/kg) or vehicle (ethanol : cremophor : saline; 1 : 1 : 18). The dose of SB366791 was based on evidence demonstrating its ability to antagonize the hypothermic effects of AM404, in a manner similar to the TRPV1 antagonist, capsazepine.31 Recent studies have demonstrated that PPARs represent a novel target for cannabinoids [for reviews see refs 32,33]. The dose and route of administration of GW9662 were based on studies demonstrating its PPARγ antagonistic activity in vivo.34–36 Rats received the above agents 1 hr before an i.p. injection of either LPS (100 μg/kg) or saline vehicle (sterile 0·89% NaCl) administered at a volume of 1 ml/kg. Blood samples were taken 2 hr after administration of LPS or saline; plasma was obtained as previously described and stored at −80° until cytokine determination.
Cytokine determinations
Plasma TNF-α, IL-1β and IL-6 concentrations were determined using specific rat enzyme-linked immunosorbent assays performed using antibodies and standards obtained from R & D Systems, Abington, UK as previously described.26 Briefly, flat-bottomed 96-well Maxisorp microtitre plates (Nunc, Weisbaden, Germany) were coated with goat or mouse anti-rat cytokine antibodies [0·8–4 μg/ml in phosphate-buffered saline (PBS): NaCl 137 mm, KCl 2·7 mm, Na2HPO4 8·1 mm, KH2PO4 1·5 mm; pH 7·4] for 20 hr at 22°. Plates were then washed three times with wash/dilution buffer (0·05% Tween-20 in PBS, pH 7·4) and blocked at room temperature for 1 hr using reagent diluent (1% bovine serum albumin in PBS, pH 7·4). Following three washes, 100-μl aliquots of samples or standards (0–5000 pg/ml) were added and plates were incubated at 22° for 2 hr. After three washes, 100 μl of specific biotinylated anti-goat or anti-mouse antibody (1 : 1000 or 1 : 2000 dilution in wash buffer containing 1% goat serum, Sigma, Dublin, Ireland) was added to each well. A further incubation was carried out for 1 hr at 22°. After three washes, 100 μl horseradish peroxidase conjugated to streptavidin (1 : 200 dilution in reagent diluent) was added to each well and plates were incubated at 22° for 20 min. Following three washes, 100 μl tetramethylbenzidine substrate solution was added per well and the plates were incubated for 20 min at 22°. At the end of the incubation period, 50 μl of 1 m H2SO4 was added per well to stop the reaction and to facilitate colour development. Absorbance was read immediately at 450 nm on a microtitre plate reader (ELx 800, Bio-Tek Instruments, Leicestershire, UK). Cytokine levels in plasma are expressed as pg/ml.
Drug preparation
URB597 (cyclohexylcarbamic acid 3′-carbamoyl-biphenyl-3-yl ester) (BioMol International, Exeter, UK), AM251 [N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamid], AM630 {6-iodo-2-methyl-1-[2-(4-morpholinyl)ethyl]-1H-indol-3-yl (4-methoxyphenyl) methanone}, AM404 [N-(4-hydroxyphenyl)-5Z,8Z,11Z,14Z-eicosatetraenamide], SB366791 (4′-chloro-3-methoxycinnamanilide) and GW9662 (2-chloro-5-nitro-N-phenylbenzamide) (Tocris, Bristol, UK) were dissolved in 100% ethanol. Surfactant (Cremophor, Sigma) and sterile saline (0·89% NaCl) were then added to produce a final concentration of either 0·3 mg/ml of URB597, 1·5 mg/ml of AM251 and AM630, 2·5, 5 and 10 mg/ml of AM404 or 1 mg/ml of SB366791 and GW9662 in vehicle (ethanol : cremophor : saline at 1 : 1 : 18). Lipopolysaccharide (Escherichia coli serotype 0111:B4, Sigma) was dissolved in sterile saline to produce a final volume of 100 μg/ml. Drugs or corresponding vehicle were administered i.p. in a volume of 1 or 2 ml/kg.
Data analysis
All data are presented as means ± SEM. Cytokine concentrations were analysed by one-way analysis of variance using spss (version 12.0.1), followed by Student–Newman–Keuls (SNK) or Fisher’s least significant difference (FLSD) post hoc test where appropriate. The level of significance was set at P < 0·05.
Results
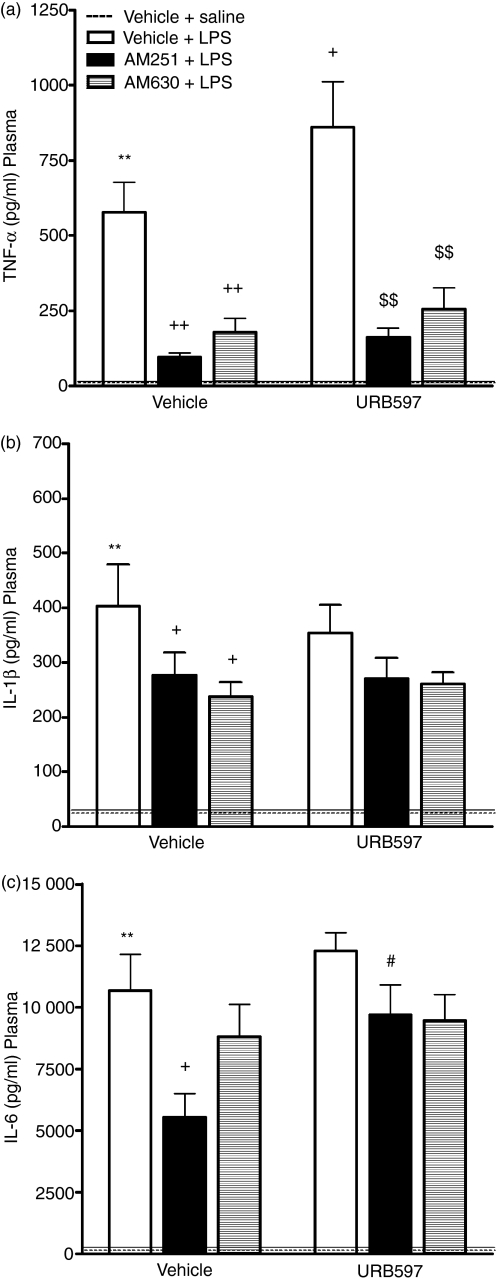
URB597 potentiates the LPS-induced increase in TNF-α, attenuation by AM251 and AM630
The effects of URB597, AM251 and AM630, alone and in combination, on plasma levels of TNF-α, IL-1β and IL-6 following LPS administration are shown in Fig. 1 together with the results of statistical analysis. Administration of LPS induced a significant increase in plasma TNF-α, IL-1β and IL-6 when compared with saline-treated controls. The FAAH inhibitor, URB597, significantly augmented the LPS-induced increase in plasma TNF-α levels, an effect attenuated by both the CB1 and CB2 receptor antagonists AM251 and AM630, respectively (Fig. 1a). URB597 did not alter LPS-induced increases in plasma IL-1β (Fig. 1b) or IL-6 (Fig. 1c). The CB1 receptor antagonist, AM251, alone significantly attenuated the LPS-induced increase in plasma TNF-α, IL-1β and IL-6. Coadministration of URB597 and AM251 prevented the AM251-induced decrease in IL-6 following LPS administration. In addition, LPS-induced production of plasma TNF-α and IL-1β was reduced by the CB2 receptor antagonist, AM630.
Figure 1.
The effect of URB597, AM251 and AM630 on lipopolysaccharide (LPS) -induced cytokine levels. URB597 potentiates LPS-induced increase in plasma tumour necrosis factor-α (TNF-α) levels, an effect attenuated by AM251 and AM630: (a) TNF-α: F6,51 = 15·98 P < 0·001 (SNK). AM251 and AM630 attenuate LPS-induced increase in interleukin-1β (IL-1β) in the plasma: (b) IL-1β: F6,52 = 6·944 P < 0·001 (FLSD). AM251 reduced LPS-induced IL-6 levels in plasma, URB597 attenuated this effect: (c) IL-6: F6,52 = 13·730 P < 0·001 (SNK). Data expressed as means + SEM (n = 7 to n = 9 per group). **P < 0·01 for Vehicle–Vehicle + LPS versus Vehicle–Vehicle + Saline (dashed line). +P < 0·05, ++P < 0·01 versus Vehicle–Vehicle + LPS. $$P < 0·01 versus URB597-Vehicle + LPS. #P < 0·05 versus Vehicle-AM251 + LPS.
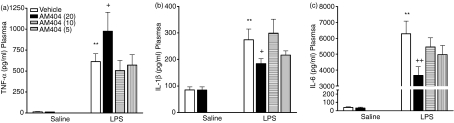
AM404 potentiates the LPS-induced increase in TNF-α while concurrently reducing IL-1β and IL-6 plasma levels
LPS administration significantly increased plasma TNF-α, IL-1β and IL-6 when compared with saline-treated controls (Fig. 2). The anandamide transport inhibitor, AM404, administered alone, did not alter plasma cytokine levels when compared with vehicle-treated controls. However, 20 mg/kg AM404 augmented the LPS-induced increase in plasma TNF-α (Fig. 2a), while concurrently reducing IL-1β (Fig. 2b) and IL-6 (Fig. 2c). Neither 5 nor 10 mg/kg of AM404 significantly affected LPS-induced increases in plasma TNF-α, IL-1β and IL-6.
Figure 2.
Effects of AM404 on lipopolysaccharide (LPS)-induced plasma cytokine levels. AM404 (20 mg/kg) potentiates LPS-induced tumour necrosis factor-α (TNF-α) levels while concurrently attenuating LPS-induced interleukin-1β (IL-1β) and IL-6 levels in plasma. (a) TNF-α: F5,46 = 10·780 P < 0·001 (SNK); (b) IL-1β: F5,47 = 9·409 P < 0·001 (FLSD); (c) IL-6: F5,46 = 32·129 P < 0·001 (SNK). Data expressed as means + SEM (n = 7 to n = 9 per group). **P < 0·01 for Vehicle + LPS versus Vehicle + Saline. +P < 0·05, ++P < 0·01 versus Vehicle + LPS. AM404 (5) = 5 mg/kg; AM404 (10) = 10 mg/kg; AM404 (20) = 20 mg/kg.
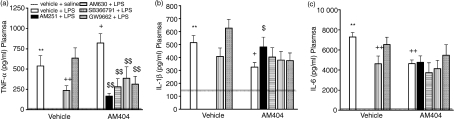
AM404 potentiates the LPS-induced increase in TNF-α, attenuation by AM251, AM630, SB366791 and GW9662
The effects of AM404 alone and in combination with the CB1 and CB2 receptor antagonists, AM251 and AM630 respectively, the TRPV1 antagonist, SB366791, and the PPARγ antagonist, GW9662, on plasma TNF-α, IL-1β and IL-6 levels following LPS administration are shown in Fig. 3 together with the results of statistical analyses. The finding that AM404 enhances the LPS-induced increase in TNF-α was replicated, and this effect was blocked by all of the antagonists examined in this study (AM251, AM630, SB366791 and GW9662; Fig. 3a). The findings that AM404 reduces LPS-induced levels of IL-1β and IL-6 were also replicated. The decrease in LPS-induced IL-1β levels following AM404 administration was attenuated by coadministration of AM251 (Fig. 3b). The AM404-induced decrease in plasma IL-6 levels was not altered by coadministration with any of the antagonists (Fig. 3c). LPS-induced increases in plasma TNF-α and IL-6 were attenuated by the TRPV1 receptor antagonist, SB366791 (Fig. 3a and 3c). Administration of the PPARγ receptor antagonist, GW9662, did not alter LPS-induced cytokine levels.
Figure 3.
The effect of cannabinoid receptor, vanilloid receptor and peroxisome proliferator-activated receptor γ (PPARγ) antagonists on AM404-induced changes in plasma cytokine levels following lipopolysaccharide (LPS) administration. AM404 potentiation of LPS-induced tumour necrosis factor-α (TNF-α) levels was attenuated by AM251, AM630, SB366791 and GW9662: (a) TNF-α: F8,76 = 7·723 P < 0·001 (SNK). AM251 blocked the AM404 decrease in interleukin-1β (IL-1β) following LPS administration: (b) IL-1β: F8,80 = 8·800 P < 0·001 (FLSD). The transient receptor potential vanilloid receptor-1 (TRPV1) receptor antagonist SB366791 reduced LPS-induced increase in TNF and IL-6: (c) IL-6: F8,67 = 13·334 P < 0·001 (FLSD). Data expressed as means + SEM (n = 7 to n = 11 per group). **P < 0·01 for Vehicle–Vehicle + LPS versus Vehicle–Vehicle + Saline (dashed line). +P < 0·05, ++P < 0·01 versus Vehicle–Vehicle + LPS. $$P < 0·01 versus AM404–Vehicle + LPS.
Discussion
Modulation of the endocannabinoid system through inhibition of re-uptake or metabolism represents an alternative strategy for the treatment of inflammatory disorders (for reviews see refs 37,38). The present study demonstrated that enhancing endocannabinoid signalling in vivo, by inhibiting FAAH or anandamide transport, potentiates LPS-induced increases in circulating TNF-α levels. Blockade of cannabinoid receptors (CB1 and CB2), the vanilloid receptor (TRPV1) and PPARγ using selective antagonists attenuated the endocannabinoid-induced augmentation of TNF-α levels following LPS administration. In addition, the anandamide transporter inhibitor, AM404, inhibited LPS-induced increases in plasma IL-1β and IL-6 levels. The immunosuppressive effect of AM404 on IL-1β levels was attenuated by the CB1 receptor antagonist AM251. However, antagonism of CB1, CB2 or TRPV1 receptors per se also blocked LPS-induced increases in cytokine levels. These results corroborate our previous findings demonstrating that selective antagonists for CB1 and CB2 receptors can exert immunosuppressive effects in vivo,26 and extend these to include antagonism of the TRPV1 receptor.
The anti-inflammatory effects of cannabinoid receptor agonists have been demonstrated in several in vitro11–13,39 and in vivo24,26,40,41 studies. However, it is possible that whereas potent synthetic agonists exert an immunosuppressive effect, endocannabinoids such as anandamide may signal through cannabinoid receptors, vanilloid receptors and/or PPARγ to facilitate LPS-induced cytokine release in vivo. Such a mechanism of action would explain the potentiation of LPS-induced TNF-α levels observed in the present study following augmentation of anandamide levels. This theory is supported by the finding that anandamide stimulates IL-6 release from astrocytes infected with Theiler’s murine encephalomyelitis virus.15 However, other lines of evidence argue against this hypothesis. Endocannabinoids and inhibition of endocannabinoid degradation or re-uptake have been shown to reduce production of cytokines, including TNF-α, in a manner similar to that which occurs following direct activation of cannabinoid receptors with synthetic agonists.13,23,42 It has been proposed that the stimulatory versus inhibitory effects of cannabinoids on the immune system may be concentration dependent.43 This may account for the immunosuppressant effects observed with large concentrations of anandamide (in the μm range) in in vitro preparations12,13,44 when compared to the stimulatory effect of anandamide on TNF-αin vivo where levels may only reach picomolar concentrations following inhibition of FAAH16 or anandamide transport.22
Antagonism of CB1, CB2, TRPV1 or PPARγ inhibited the potentiation of LPS-induced increases in TNF-α following FAAH inhibition or anandamide transporter blockade. These results may suggest that anandamide signalling through any one of these receptor types is sufficient for LPS-induced increases in peripheral TNF-α levels. However, it should be noted that AM251, AM630 and SB366791 alone exhibited immunosuppressive properties and therefore it is possible that their attenuation of the effects of URB597 and AM404 may occur as a result of indirect physiological antagonism rather than direct competitive pharmacological blockade of the actions of endocannabinoids at these receptor targets. The immunosuppressive effect of these antagonists may supersede any facilitatory effect of URB597 or AM404 on LPS-induced increase in plasma cytokines and one cannot assume that the observed effects of these indirect cannabinoid agonists are mediated by CB1, CB2 or TRPV1. In contrast, the PPARγ antagonist, GW9662, did not affect LPS-induced TNF-α levels in the absence of AM404 but did attenuate the AM404-induced augmentation of TNF-α levels post-LPS. Many cannabinoids, including anandamide, bind directly to PPARγ, inducing transcriptional activation.9 Moreover, anandamide-induced suppression of IL-2 has been proposed to occur via activation of PPARγ.44 However, it is not known what effect anandamide-induced activation of PPARγ has on other cytokines. Although several studies indicate that activation of PPARγ with the synthetic high-affinity ligand, 15d-PGJ2, inhibits LPS-induced increases in TNF-α release from microglial cells,45,46 it has been suggested that these immunosuppressant actions may be mediated via PPARγ-independent mechanisms.47 Furthermore, administration of PPARγ ligands in vivo potentiates LPS-induced increases in plasma and brain TNF-α and IL-6 levels.47,48 PPARγ expression in cultured microglial cells is down-regulated by LPS45 an effect which may also occur in the peripheral immune system. We propose a possible mechanism of action; LPS induces an increase in endocannabinoids49–51 which, because of the downregulation of PPARγ by LPS,45 may not affect signalling through this receptor. However, pharmacological enhancement of endogenous anandamide tone following URB597 or AM404 administration may result in activation of PPARγ and potentiate the LPS-induced increase in TNF-α levels. Such a mechanism would explain why, in the present study, the PPARγ antagonist GW9662 blocked the AM404-induced potentiation of TNF-α. In addition, blockade of the CB1 receptor with the selective antagonist AM251, in the presence of enhanced endocannabinoid tone, may unmask the immunostimulatory effects of anandamide at PPARγ, resulting in the increased IL-6 levels observed in this study.
Although several theories have been proposed, the precise mechanism underlying the immunosuppressive actions of cannabinoid and vanilloid receptor antagonists remains to be elucidated. As discussed above, arguments exist for and against the theory that endocannabinoids released in response to LPS49–51 may act through the cannabinoid and vanilloid receptors to facilitate LPS-induced increases in cytokine levels. If this were the case, then blockade of CB1, CB2 and/or TRPV1 receptors should prevent the increase in circulating levels of TNF-α, IL-1β and IL-6 following LPS administration, as observed in this study. Alternatively, the immunosuppressive effects of cannabinoid and vanilloid antagonists may be the result of the unmasking of endocannabinoid actions at other receptors such as PPARs or GPR55. 9,10,44 It has also been suggested that the cannabinoid receptor antagonists, SR141716A (rimonabant) and SR144528, may act as partial agonists at CB1 and CB2 receptors respectively, when administered alone.24–26,52,53 In addition, recent evidence suggests that AM251 acts as an agonist at the newly classified cannabinoid receptor GPR55.10 However, pharmacological agents acting selectively at GPR55 are not yet commercially available. Further studies are required to determine if the immunosuppressive activity observed by cannabinoid and vanilloid receptor antagonists are the result of partial agonist-like effects at their respective or alternative receptors.
Whereas both URB597 and AM404 potentiated LPS-induced increases in circulating TNF-α, only AM404 attenuated LPS-induced increases in IL-1β and IL-6. The immunosuppressive effects of AM404 reported here support other studies demonstrating that different anandamide transport inhibitors also reduce IL-1β levels ex vivo23 and in vitro.42 The differential effects of URB597 and AM404 on these cytokines may be the result of differential modulation of endocannabinoid levels or direct or indirect action of these compounds at alternative targets. For example, AM404 does not affect levels of the endogenous cannabinoids N-oleoyl ethanolamide 10 (OEA) and N-palmitoyl ethanolamide (PEA), which are enhanced following FAAH inhibition with URB597.16,17 In addition to inhibiting anandamide transport, AM404 also acts as a full agonist at TRPV1.54 However, the failure of the TRPV1 antagonist, SB366791, to block the immunosuppressive effects of AM404 on IL-1β and IL-6 suggests that the effects observed were not TRPV1-mediated. The immunosuppressive effects of AM404 on IL-1β were, however, blocked by AM251, reaffirming the role of the CB1 receptor in modulating peripheral cytokine levels.24–26 None of the antagonists used in the present study attenuated the AM404-induced decrease in IL-6 and further work is needed to determine the receptor mechanisms underlying this effect.
In summary, the present study demonstrated that inhibition of anandamide degradation or uptake potentiates LPS-induced increases in circulating TNF-α levels, an effect which may be mediated by PPARγ. In comparison, the immunosuppressive effects of the anandamide transport inhibitor AM404 on LPS-induced IL-1β appear to be CB1 receptor dependent. The receptor mechanisms mediating the immunosuppressive effect of AM404 on LPS-induced IL-6 remain to be determined. In addition, CB1, CB2 and TRPV1 receptor antagonists alone inhibited LPS-induced increases in plasma cytokine levels, results which corroborate previous studies in our laboratory26 and highlight the complexity of cannabinoid–immune interactions. Several diseases have been associated with increased proinflammatory cytokine levels including rheumatoid arthritis, diabetes, human immunodeficiency virus infection, chronic pain and a number of neurological diseases. Uncovering the mechanisms underpinning cannabinoid-mediated modulation of immune system physiology may provide alternative treatment strategies for many of these diseases.
Acknowledgments
The technical assistance of Danny Kerr, Michael Diamond and Kieran Rea is gratefully acknowledged. This work was supported by a grant from the Irish Health Research Board.
Abbreviations
- FAAH
fatty acid amide hydrolase
- IL-1β
interleukin-1β
- i.p.
intraperitoneal
- LPS
lipopolysaccharide
- PPAR
peroxisome proliferator-activated receptor
- TNF-α
tumour necrosis factor-α
- TRPV1
transient receptor potential vanilloid receptor-1
References
- 1.Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–4. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- 2.Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–5. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- 3.Mechoulam R, Fride E, Di Marzo V. Endocannabinoids. Eur J Pharmacol. 1998;359:1–18. doi: 10.1016/s0014-2999(98)00649-9. [DOI] [PubMed] [Google Scholar]
- 4.Beltramo M, Stella N, Calignano A, Lin SY, Makriyannis A, Piomelli D. Functional role of high-affinity anandamide transport, as revealed by selective inhibition. Science. 1997;277:1094–7. doi: 10.1126/science.277.5329.1094. [DOI] [PubMed] [Google Scholar]
- 5.Hillard CJ, Edgemond WS, Jarrahian A, Campbell WB. Accumulation of N-arachidonoylethanolamine (anandamide) into cerebellar granule cells occurs via facilitated diffusion. J Neurochem. 1997;69:631–8. doi: 10.1046/j.1471-4159.1997.69020631.x. [DOI] [PubMed] [Google Scholar]
- 6.Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–7. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- 7.Dinh TP, Carpenter D, Leslie FM, Freund TF, Katona I, Sensi SL, Kathuria S, Piomelli D. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc Natl Acad Sci USA. 2002;99:10819–24. doi: 10.1073/pnas.152334899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sorgard M, Di Marzo V, Julius D, Hogestatt ED. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–7. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]
- 9.Bouaboula M, Hilairet S, Marchand J, Fajas L, Le Fur G, Casellas P. Anandamide induced PPARγ transcriptional activation and 3T3-L1 preadipocyte differentiation. Eur J Pharmacol. 2005;517:174–81. doi: 10.1016/j.ejphar.2005.05.032. [DOI] [PubMed] [Google Scholar]
- 10.Ryberg E, Larsson N, Sjogren S, et al. The orphan receptor GPR55 is a novel cannabinoid receptor. Br J Pharmacol. 2007;152:1092–101. doi: 10.1038/sj.bjp.0707460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Puffenbarger RA, Boothe AC, Cabral GA. Cannabinoids inhibit LPS-inducible cytokine mRNA expression in rat microglial cells. Glia. 2000;29:58–69. [PubMed] [Google Scholar]
- 12.Chang YH, Lee ST, Lin WW. Effects of cannabinoids on LPS-stimulated inflammatory mediator release from macrophages: involvement of eicosanoids. J Cell Biochem. 2001;81:715–23. doi: 10.1002/jcb.1103. [DOI] [PubMed] [Google Scholar]
- 13.Facchinetti F, Del Giudice E, Furegato S, Passarotto M, Leon A. Cannabinoids ablate release of TNFalpha in rat microglial cells stimulated with lipopolysaccharide. Glia. 2003;41:161–8. doi: 10.1002/glia.10177. [DOI] [PubMed] [Google Scholar]
- 14.Schwarz H, Blanco FJ, Lotz M. Anandamide, an endogenous cannabinoid receptor agonist inhibits lymphocyte proliferation and induces apoptosis. J Neuroimmunol. 1994;55:107–15. doi: 10.1016/0165-5728(94)90152-x. [DOI] [PubMed] [Google Scholar]
- 15.Molina-Holgado F, Molina-Holgado E, Guaza C. The endogenous cannabinoid anandamide potentiates interleukin-6 production by astrocytes infected with Theiler's murine encephalomyelitis virus by a receptor-mediated pathway. FEBS Lett. 1998;433:139–42. doi: 10.1016/s0014-5793(98)00851-5. [DOI] [PubMed] [Google Scholar]
- 16.Kathuria S, Gaetani S, Fegley D, et al. Modulation of anxiety through blockade of anandamide hydrolysis. Nat Med. 2003;9:76–81. doi: 10.1038/nm803. [DOI] [PubMed] [Google Scholar]
- 17.Fegley D, Gaetani S, Duranti A, Tontini A, Mor M, Tarzia G, Piomelli D. Characterization of the fatty acid amide hydrolase inhibitor cyclohexyl carbamic acid 3′-carbamoyl-biphenyl-3-yl ester (URB597): effects on anandamide and oleoylethanolamide deactivation. J Pharmacol Exp Ther. 2005;313:352–8. doi: 10.1124/jpet.104.078980. [DOI] [PubMed] [Google Scholar]
- 18.Piomelli D, Tarzia G, Duranti A, et al. Pharmacological profile of the selective FAAH inhibitor KDS-4103 (URB597) CNS Drug Rev. 2006;12:21–38. doi: 10.1111/j.1527-3458.2006.00021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holt S, Comelli F, Costa B, Fowler CJ. Inhibitors of fatty acid amide hydrolase reduce carrageenan-induced hind paw inflammation in pentobarbital-treated mice: comparison with indomethacin and possible involvement of cannabinoid receptors. Br J Pharmacol. 2005;146:467–76. doi: 10.1038/sj.bjp.0706348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jayamanne A, Greenwood R, Mitchell VA, Aslan S, Piomelli D, Vaughan CW. Actions of the FAAH inhibitor URB597 in neuropathic and inflammatory chronic pain models. Br J Pharmacol. 2006;147:281–8. doi: 10.1038/sj.bjp.0706510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tham CS, Whitaker J, Luo L, Webb M. Inhibition of microglial fatty acid amide hydrolase modulates LPS stimulated release of inflammatory mediators. FEBS Lett. 2007;581:2899–904. doi: 10.1016/j.febslet.2007.05.037. [DOI] [PubMed] [Google Scholar]
- 22.Giuffrida A, Rodriguez de Fonseca F, Nava F, Loubet-Lescoulie P, Piomelli D. Elevated circulating levels of anandamide after administration of the transport inhibitor, AM404. Eur J Pharmacol. 2000;408:161–8. doi: 10.1016/s0014-2999(00)00786-x. [DOI] [PubMed] [Google Scholar]
- 23.Mestre L, Correa F, Arevalo-Martin A, Molina-Holgado E, Valenti M, Ortar G, Di Marzo V, Guaza C. Pharmacological modulation of the endocannabinoid system in a viral model of multiple sclerosis. J Neurochem. 2005;92:1327–39. doi: 10.1111/j.1471-4159.2004.02979.x. [DOI] [PubMed] [Google Scholar]
- 24.Smith SR, Terminelli C, Denhardt G. Effects of cannabinoid receptor agonist and antagonist ligands on production of inflammatory cytokines and anti-inflammatory interleukin-10 in endotoxemic mice. J Pharmacol Exp Ther. 2000;293:136–50. [PubMed] [Google Scholar]
- 25.Croci T, Landi M, Galzin AM, Marini P. Role of cannabinoid CB1 receptors and tumor necrosis factor-alpha in the gut and systemic anti-inflammatory activity of SR 141716 (rimonabant) in rodents. Br J Pharmacol. 2003;140:115–22. doi: 10.1038/sj.bjp.0705412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roche M, Diamond M, Kelly JP, Finn DP. In vivo modulation of LPS-induced alterations in brain and peripheral cytokines and HPA axis activity by cannabinoids. J Neuroimmunol. 2006;181:57–67. doi: 10.1016/j.jneuroim.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 27.Moezi L, Gaskari SA, Liu H, Baik SK, Dehpour AR, Lee SS. Anandamide mediates hyperdynamic circulation in cirrhotic rats via CB(1) and VR(1) receptors. Br J Pharmacol. 2006;149:898–908. doi: 10.1038/sj.bjp.0706928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hama A, Sagen J. Antinociceptive effect of cannabinoid agonist WIN 55,212-2 in rats with a spinal cord injury. Exp Neurol. 2007;204:454–7. doi: 10.1016/j.expneurol.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roche M, Diamond M, Lonergan I, Keeney G, Kelly JP, Finn DP. The effects of acute lipopolysaccharide injection on levels of pro- and anti-inflammatory cytokines in discrete rat brain regions. J Psychopharmacol. 2005;19:A37. [Google Scholar]
- 30.Costa B, Siniscalco D, Trovato AE, et al. AM404, an inhibitor of anandamide uptake, prevents pain behaviour and modulates cytokine and apoptotic pathways in a rat model of neuropathic pain. Br J Pharmacol. 2006;148:1022–32. doi: 10.1038/sj.bjp.0706798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Varga A, Nemeth J, Szabo A, et al. Effects of the novel TRPV1 receptor antagonist SB366791 in vitro and in vivo in the rat. Neurosci Lett. 2005;385:137–42. doi: 10.1016/j.neulet.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 32.Burstein S. PPAR-gamma: a nuclear receptor with affinity for cannabinoids. Life Sci. 2005;77:1674–84. doi: 10.1016/j.lfs.2005.05.039. [DOI] [PubMed] [Google Scholar]
- 33.O'Sullivan S E. Cannabinoids go nuclear: evidence for activation of peroxisome proliferator-activated receptors. Br J Pharmacol. 2007;152:576–82. doi: 10.1038/sj.bjp.0707423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Collin M, Patel NS, Dugo L, Thiemermann C. Role of peroxisome proliferator-activated receptor-gamma in the protection afforded by 15-deoxydelta12,14 prostaglandin J2 against the multiple organ failure caused by endotoxin. Crit Care Med. 2004;32:826–31. doi: 10.1097/01.ccm.0000114821.25573.e7. [DOI] [PubMed] [Google Scholar]
- 35.Shimizu T, Szalay L, Hsieh YC, Suzuki T, Choudhry MA, Bland KI, Chaudry IH. A role of PPAR-gamma in androstenediol-mediated salutary effects on cardiac function following trauma-hemorrhage. Ann Surg. 2006;244:131–8. doi: 10.1097/01.sla.0000217709.00863.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park SW, Yi JH, Miranpuri G, Satriotomo I, Bowen K, Resnick DK, Vemuganti R. Thiazolidinedione class of peroxisome proliferator-activated receptor gamma agonists prevents neuronal damage, motor dysfunction, myelin loss, neuropathic pain, and inflammation after spinal cord injury in adult rats. J Pharmacol Exp Ther. 2007;320:1002–12. doi: 10.1124/jpet.106.113472. [DOI] [PubMed] [Google Scholar]
- 37.Pertwee RG. The therapeutic potential of drugs that target cannabinoid receptors or modulate the tissue levels or actions of endocannabinoids. AAPS J. 2005;7:E625–54. doi: 10.1208/aapsj070364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maccarrone M. Fatty acid amide hydrolase: a potential target for next generation therapeutics. Curr Pharm Des. 2006;12:759–72. doi: 10.2174/138161206775474279. [DOI] [PubMed] [Google Scholar]
- 39.Molina-Holgado F, Pinteaux E, Moore JD, Molina-Holgado E, Guaza C, Gibson RM, Rothwell NJ. Endogenous interleukin-1 receptor antagonist mediates anti-inflammatory and neuroprotective actions of cannabinoids in neurons and glia. J Neurosci. 2003;23:6470–4. doi: 10.1523/JNEUROSCI.23-16-06470.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gallily R, Yamin A, Waksmann Y, et al. Protection against septic shock and suppression of tumor necrosis factor alpha and nitric oxide production by dexanabinol (HU-211), a nonpsychotropic cannabinoid. J Pharmacol Exp Ther. 1997;283:918–24. [PubMed] [Google Scholar]
- 41.Panikashvili D, Simeonidou C, Ben-Shabat S, Hanus L, Breuer A, Mechoulam R, Shohami E. An endogenous cannabinoid (2-AG) is neuroprotective after brain injury. Nature. 2001;413:527–31. doi: 10.1038/35097089. [DOI] [PubMed] [Google Scholar]
- 42.Ortega-Gutierrez S, Molina-Holgado E, Guaza C. Effect of anandamide uptake inhibition in the production of nitric oxide and in the release of cytokines in astrocyte cultures. Glia. 2005;52:163–8. doi: 10.1002/glia.20229. [DOI] [PubMed] [Google Scholar]
- 43.Berdyshev EV, Boichot E, Germain N, Allain N, Anger JP, Lagente V. Influence of fatty acid ethanolamides and delta9-tetrahydrocannabinol on cytokine and arachidonate release by mononuclear cells. Eur J Pharmacol. 1997;330:231–40. doi: 10.1016/s0014-2999(97)01007-8. [DOI] [PubMed] [Google Scholar]
- 44.Rockwell CE, Kaminski NE. A cyclooxygenase metabolite of anandamide causes inhibition of interleukin-2 secretion in murine splenocytes. J Pharmacol Exp Ther. 2004;311:683–90. doi: 10.1124/jpet.104.065524. [DOI] [PubMed] [Google Scholar]
- 45.Bernardo A, Levi G, Minghetti L. Role of the peroxisome proliferator-activated receptor-gamma (PPAR-gamma) and its natural ligand 15-deoxy-Delta12, 14-prostaglandin J2 in the regulation of microglial functions. Eur J Neurosci. 2000;12:2215–23. doi: 10.1046/j.1460-9568.2000.00110.x. [DOI] [PubMed] [Google Scholar]
- 46.Storer PD, Xu J, Chavis JA, Drew PD. Cyclopentenone prostaglandins PGA2 and 15-deoxy-delta12,14 PGJ2 suppress activation of murine microglia and astrocytes: implications for multiple sclerosis. J Neurosci Res. 2005;80:66–74. doi: 10.1002/jnr.20413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thieringer R, Fenyk-Melody JE, Le Grand CB, et al. Activation of peroxisome proliferator-activated receptor gamma does not inhibit IL-6 or TNF-alpha responses of macrophages to lipopolysaccharide in vitro or in vivo. J Immunol. 2000;164:1046–54. doi: 10.4049/jimmunol.164.2.1046. [DOI] [PubMed] [Google Scholar]
- 48.Gelinas DS, Lambermon MH, McLaurin J. Ciglitazone increases basal cytokine expression in the central nervous system of adult rats. Brain Res. 2005;1034:139–46. doi: 10.1016/j.brainres.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 49.Di Marzo V, Bisogno T, De Petrocellis L, Melck D, Orlando P, Wagner JA, Kunos G. Biosynthesis and inactivation of the endocannabinoid 2-arachidonoylglycerol in circulating and tumoral macrophages. Eur J Biochem. 1999;264:258–67. doi: 10.1046/j.1432-1327.1999.00631.x. [DOI] [PubMed] [Google Scholar]
- 50.Maccarrone M, De Petrocellis L, Bari M, Fezza F, Salvati S, Di Marzo V, Finazzi-Agro A. Lipopolysaccharide downregulates fatty acid amide hydrolase expression and increases anandamide levels in human peripheral lymphocytes. Arch Biochem Biophys. 2001;393:321–8. doi: 10.1006/abbi.2001.2500. [DOI] [PubMed] [Google Scholar]
- 51.Maccarrone M, Falciglia K, Di Rienzo M, Finazzi-Agro A. Endocannabinoids, hormone–cytokine networks and human fertility. Prostaglandins Leukotr Essent Fatty Acids. 2002;66:309–17. doi: 10.1054/plef.2001.0354. [DOI] [PubMed] [Google Scholar]
- 52.Smith SR, Terminelli C, Denhardt G. Modulation of cytokine responses in Corynebacterium parvum-primed endotoxemic mice by centrally administered cannabinoid ligands. Eur J Pharmacol. 2001;425:73–83. doi: 10.1016/s0014-2999(01)01142-6. [DOI] [PubMed] [Google Scholar]
- 53.Krylatov AV, Maslov LN, Lasukova OV, Pertwee RG. Cannabinoid receptor antagonists SR141716 and SR144528 exhibit properties of partial agonists in experiments on isolated perfused rat heart. Bull Exp Biol Med. 2005;139:558–61. doi: 10.1007/s10517-005-0344-9. [DOI] [PubMed] [Google Scholar]
- 54.Zygmunt PM, Chuang H, Movahed P, Julius D, Hogestatt ED. The anandamide transport inhibitor AM404 activates vanilloid receptors. Eur J Pharmacol. 2000;396:39–42. doi: 10.1016/s0014-2999(00)00207-7. [DOI] [PubMed] [Google Scholar]