Abstract
Murine γδ T cells participate in the innate immune response against infection by an intracellular pathogen Listeria monocytogenes. Vδ1+γδ T cells coexpressing Vγ6 are a major γδ T-cell subpopulation induced at an early stage of L. monocytogenes infection in the livers of infected mice. To investigate the protective role of the Vγ6/Vδ1+γδ T cells against L. monocytogenes infection, Vδ1 gene-deficient (Vδ1−/−) mice were analysed because these mice selectively lacked a Vγ6/Vδ1+γδ T-cell subpopulation in the L. monocytogenes-infected liver. The Vδ1−/− mice showed increased bacterial burden in the liver and spleen, and decreased survival rate at an early stage of L. monocytogenes infection when compared to wild-type mice. Histological examination showed abscess-like lesions and unorganized distribution of macrophages in the liver of the Vδ1−/− mice but not in the wild-type mice after L. monocytogenes infection. The Vγ6/Vδ1+γδ T cells produced interferon-γ and interleukin-17A. All the results suggest that murine Vγ6/Vδ1+γδ T cells control the innate protective response against L. monocytogenes infection through production of the proinflammatory cytokines interferon-γ and interleukin-17A in the infected liver.
Keywords: interferon-γ, interleukin-17A, Listeria monocytogenes, γδ T cell
Introduction
T cells expressing the T-cell receptor (TCR)-γδ heterodimer belong to a unique T-cell lineage distinct from conventional T cells expressing the TCR-αβ heterodimer.1 A restricted number of TCR Vγ and Vδ genes is available to encode functional TCR-γδ, suggesting that the γδ T cells recognize a restricted number of ligands. However, the ligand specificity of the majority of γδ T cells has not been determined.2γδ T cells can be divided into functional subpopulations, which are similar to the T helper type 1 (Th1), Th2, Th3, Th17 and cytotoxic T cells of TCR-αβ T cells,2–6 while the role of the γδ T cells in in vivo immune responses is not well characterized.
The γδ T cells have been reported to participate in immune responses against various infections.7Listeria monocytogenes, an intracellular bacterium, has been extensively used in analyses of the role of γδ T cells, and the importance of γδ T cells in innate protective immunity against this infection is well established.8–10 Murine γδ T cells induced by the L. monocytogenes infection can be divided into three subpopulations, Vγ1/Vδ6+,11–13 Vγ4+,12 and Vγ6/Vδ1+14,15γδ T cells, according to their TCR Vγ/Vδ expression. The protective role of the Vγ1+γδ T cells has been demonstrated,12 although another report indicated an opposite role for Vγ1+ T cells.13 Vγ1+γδ T-cell-mediated killing of activated macrophages has also been demonstrated.16 Depletion of Vγ4+γδ T cells has no significant effect on protective immunity against L. monocytogenes infection, suggesting that Vγ4 does not have a significant role in protective immunity.13
To further clarify the repertoire-specific function of the γδ T cells in protection against L. monocytogenes infection, we analysed Vδ1 gene-deficient (Vδ1−/−) mice.17 The results demonstrated that the Vδ1−/− mice selectively lacked Vγ6/Vδ1+γδ T cells but retained Vγ1+ and Vγ4+γδ T cells in the liver after L. monocytogenes infection, and that the Vγ6/Vδ1+γδ T cells had an important role in innate protective immunity against L. monocytogenes infection.
Materials and methods
Animals
Wild-type C57BL/6 mice were purchased from Japan SLC (Shizuoka, Japan). Vδ1−/− mice17 were backcrossed more than eight times to C57BL/6 mice. The mice were maintained in a conventional environment, and were used for experiments at 8–10 weeks of age. Experiments were conducted according to the Institutional Ethical Guidelines for Animal Experiments and the Safety Guideline for Gene Manipulation Experiments of the University of the Ryukyus.
Microorganisms and bacterial infection
Listeria monocytogenes strain EGD was inoculated into C57BL/6 mice, fresh isolates were obtained from infected spleens, grown in tryptic soy broth (Difco, Detroit, MI), resuspended in phosphate-buffered saline, and stored at −80° in small samples until use. Mice were infected by intraperitoneal (i.p.) inoculation of 5 × 104 colony-forming units of L. monocytogenes [which corresponds to 1/10 of the 50% lethal dose (LD50) for wild-type C57BL/6 mice] except for the analysis of survival rate, for which 2·5 × 105 colony-forming units of L. monocytogenes was inoculated.
Cell preparation
Liver mononuclear cells were prepared as described previously.18 To enrich TCR-γδ T cells, the cells were passed through nylon wool columns, then separated using a magnetic cell sorter system (autoMACS, Miltenyi, Bergisch Gladbach, Germany) by using biotin- or fluorescein isothiocyanate (FITC)-conjugated anti-TCR Cδ monoclonal antibody (mAb; GL-3, Becton Dickinson, San Jose, CA) and streptavidin or anti-FITC microbeads (Miltenyi), respectively.
Gene expression analysis by reverse transcription–polymerase chain reaction (RT-PCR)
Using Trizol reagent (Invitrogen, Carlsbad, CA), total RNA was extracted. First-strand cDNA was synthesized, and then amplified by PCR using Taq polymerase (Takara Shuzo, Kyoto, Japan) with TCR Vγ or Vδ sense primers and Cγ or Cδ antisense primers, as described previously.19 The following primers were used (nomenclature of Vγ genes is according to Heilig and Tonegawa20): Cγ, CTT ATG GAG ATT TGT TTC AGC; Vγ1-3, ACA CAG CTA TAC ATT GGT AC; Vγ2, CGG CAA AAA ACA AAT CAA CAG: Vγ4, TGT CCT TGC AAC CCC TAC CC; Vγ5, TGT GCA CTG GTA CCA ACT GA; Vγ6, CTC CAA AGA ATG CTG TGT AG; Vγ7, AAG CTA GAG GGG TCC TCT GC; Cδ, CGA ATT CCA CAA TCT TCT TG; Vδ1, ATT CAG AAG GCA ACA ATG AAA G; Vδ2, AGT TCC CTG CAG ATC CAA GC; Vδ3, TTC CTG GCT ATT GCC TCT GAC; Vδ4, CCG CTT CTC TGT GAA CTT CC; Vδ5, CAG ATC CTT CCA GTT CAT CC; Vδ6, CTT AGT GGA GAG ATG GTT TT; Vδ7, CGC AGA GCT GCA GTG TAA CT; and Vδ8, AAG GAA GAT GGA CGA TTC AC. The Vγ1-3 primer amplifies the Vγ1, Vγ2 and Vγ3 genes, whereas the Vγ2 primer amplifies the Vγ2 gene but not the Vγ1 gene. Since γδ T cells with functional Vγ3 expression have not yet been documented, we consider that the Vγ1-3 primer detects TCR of Vγ1+ and Vγ2+γδ T cells. The PCR products were electrophoresed through 1·8% agarose gel, stained with ethidium bromide, and photographed using the Gel-Documentation system (BioRad, Hercules, CA).
Flow cytometry (FCM)
Cells were stained with mAbs against leucocyte surface molecules and cytokines, and analysed by FCM. The following mAbs were used to detect surface molecules: FITC-conjugated anti-CD3, anti-Gr1 (Becton Dickinson), and anti-TCR Cβ (Becton Dickinson) mAbs, phycoerythrin-conjugated anti-TCR Cδ, anti-CD11b (Caltag, Burlingame, CA), anti-CD4 (Becton Dickinson), anti-CD8 (Becton Dickinson) and anti-NK1.1 (Becton Dickinson) mAbs. After staining, cells were analysed using a FACSCalibur system (Becton Dickinson), and the proportions of macrophages (Gr1− CD11b+), neutrophils (Gr1+ CD11b+), TCR γδ T cells (CD3+ TCR Cδ+), CD4+ T cells (CD4+ TCR Cβ+), CD8+ T cells (CD8+ TCR Cβ+) and natural killer T cells (NK.1+ TCR Cβ+) were determined. The absolute number of each fraction was calculated using absolute number of liver mononuclear cells per mouse and the ratio of each cell population. To detect cytokine expression of γδ T cells, the γδ T cells were enriched with biotin-conjugated anti-TCR Cδ mAb and autoMACS, cultured with 1 μg/ml calcium ionophore, 25 ng/ml phorbol 12-myristate 13-acetate, and brefeldin A for 4 hr, stained with mAb against surface antigens and then treated with Cytofix/Cytoperm solution (Becton Dickinson) according to the manufacturer’s instructions followed by intracellular cytokine staining with phycoerythrin-conjugated anti-interleukin-17A (IL-17A), or phycoerythrin-conjugated anti-interferon-γ (IFN-γ) mAbs. To detect biotin-conjugated anti-TCR Cδ mAb, allophycocyanin-conjugated streptavidin was used. FITC-conjugated anti-TCR Vγ1 (clone 2.1121), anti-TCR Vγ4 (Becton Dickinson), anti-TCR Vγ5 (Becton Dickinson) and anti-TCR Vγ7 (clone F2.6722) mAbs were mixed (anti-Vγ mAb mixture) and used to detect Vγ6+ and Vγ2+γδ T cells as anti-Vγ mAb mixture-negative TCR Cδ-positive cells.
Bacteria counts in organs
The L. monocytogenes-infected mice were killed on day 5 of the infection, the liver and spleen were homogenized in saline, and the homogenates were plated on nutrient agar plates containing 0·4% glucose to calculate the number of bacteria in each organ.
Histopathology
The livers of L. monocytogenes-infected mice were fixed in buffered formalin on day 5 of the infection, and embedded in paraffin. Thin sections were prepared and stained with haematoxylin & eosin. The stained sections were examined under a BX41 microscope (Olympus, Tokyo, Japan) equipped with 4×/0·13 and 20×/0·50 objectives. Images were acquired with a DP70 digital camera and dp software (Olympus). The liver was also embedded in optimal cutting temperature compound (Sakura, Tokyo, Japan), and frozen in dry ice–acetone. Thin sections were prepared with a cryostat, stained with allophycocyanin-conjugated anti-CD3 mAb and Alexa 488-conjugated anti-CD11b mAb (Becton Dickinson), and analysed using a Radiance 2100 (BioRad) confocal laser scanning microscope (CF-LSM) equipped with a 20×/0·70 objective. The images of CF-LSM were acquired with LaserSharp 2000 software, (BioRad) and merged using Adobe Photoshop software (Adobe, San Jose, CA).
Statistics
Data were statistically evaluated using Student’s t-test and Statwork software (Cricket Software, Philadelphia, PA). The survival rate was analysed by the Kaplan–Meier method and statistically evaluated by Log rank test using Statcel 2 software (MOS, Saitama, Japan). A P value < 0·05 was considered to indicate statistical significance.
Results
Increase of γδ T cells expressing TCR Vγ6/Vδ1 in the liver of L. monocytogenes-infected mice and lack of the γδ T-cell subpopulation in the Vδ1−/− mice
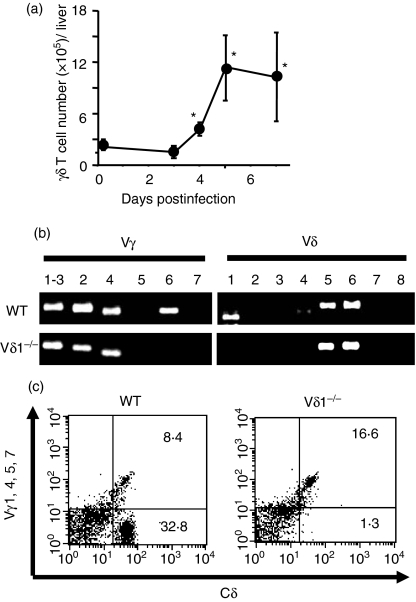
It has been reported that γδ T cells increase and participate in innate protective immunity against L. monocytogenes infection.8–10 In the liver of wild-type C57BL/6 mice, γδ T cells increased from day 4 and reached a maximum on day 5 of L. monocytogenes infection (Fig. 1a). Analysis of Vγ and Vδ gene expression by the L. monocytogenes-induced liver γδ T cells using RT-PCR showed that the liver γδ T cells expressed Vγ1 and/or Vγ2, Vγ4 and Vγ6 as Vγ genes, and Vδ1, Vδ5 and Vδ6 as Vδ genes (Fig. 1b, upper panels). The data were consistent with a previous report showing the presence of Vγ1/Vδ6+, Vγ4+, and Vγ6/Vδ1+γδ T cells in the L. monocytogenes-infected mice.11–16 When the liver γδ T cells were stained with an anti-Vγ (anti-Vγ1, anti-Vγ4, anti-Vγ5 and anti-Vγ7) mAb mixture, 50–80% of the γδ T cells were not stained (Fig. 1c, left panel), suggesting the presence of a high percentage of γδ T cells expressing Vγ6 or Vγ2.
Figure 1.
T-cell receptor (TCR) V region repertoire of the γδ T cells induced in the liver of Listeria monocytogenes-infected mice. (a) Wild-type C57BL/6 mice were intraperitoneally inoculated with L. monocytogenes, and kinetics of the liver γδ T-cell number was calculated using total liver mononuclear cell number and the ratio of the γδ T cells determined by flow cytometry (FCM). The data shown are representative of three independent experiments. (b) Vγ and Vδ gene expression of the γδ T cells were analysed by reverse transcription–polymerase chain reaction on the liver mononuclear cells from wild-type (WT) or Vδ1−/− mice on day 5 of L. monocytogenes infection. The data are representative of three independent experiments. (c) Liver γδ T cells were analysed on their expression of Vγ gene products by FCM. The liver γδ T cells were enriched from WT or Vδ1−/− mice on day 5 of L. monocytogenes intraperitoneal infection, and were stained with anti-Cδ monoclonal antibody (mAb) and anti-Vγ mAb mixture as described in the Materials and methods section. The cells in the lower right quadrant correspond to the γδ T cells that lack expression of Vγ1, Vγ4, Vγ5 and Vγ7. The experiments were repeated three to five times, and representative FCM profiles are shown.
Selective pairing of Vδ1 with Vγ5 and Vγ6 has been reported.23 Analysis using RT-PCR showed that Vγ6 expression, but not Vγ5 expression, was detected in the liver γδ T cells from L. monocytogenes-infected wild-type mice (Fig. 1b, upper panel). Analysis by FCM using anti-TCR Vγ5 mAb also failed to detect Vγ5+γδ T cells (data not shown). Therefore, we reasoned that Vδ1−/− mice selectively lack the Vγ6/Vδ1+γδ T cells in the liver γδ T cells after L. monocytogenes infection. Consistent with this theory – not only the Vδ1 gene but also the Vγ6 genes were undetectable in the liver γδ T cells of the Vδ1−/− mice while Vγ2 gene expression was not affected (Fig. 1b, lower panels). Analysis by FCM of the liver γδ T cells in the Vδ1−/− mice showed that the ratio of anti-Vγ mAb mixture-negative γδ T cells decreased to 5–15% of the γδ T cells (Fig. 1c, right panel), indicating that the majority of the anti-Vγ mAb mixture-negative γδ T cells in the L. monocytogenes-infected liver of wild-type mice were Vγ6/Vδ1+γδ T cells. All the results demonstrated that the Vγ6/Vδ1+γδ T cells formed a major subpopulation of the γδ T cells induced in the liver by L. monocytogenes infection, and that the Vδ1−/− mice selectively lacked this γδ T-cell subpopulation.
Protective role of Vγ6/Vδ1+γδ T cells against L. monocytogenes at an early stage of infection
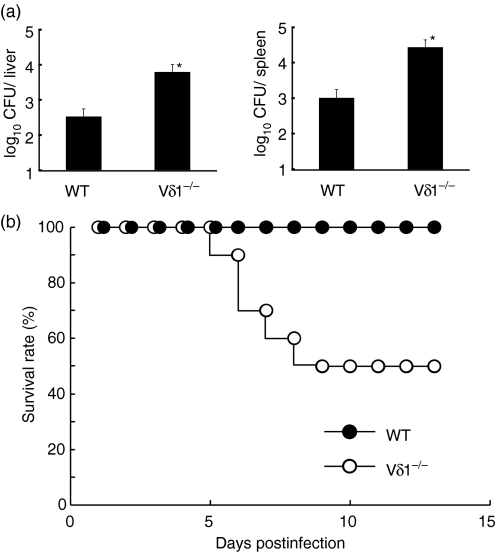
To analyse the protective role of Vγ6/Vδ1+γδ T cells against L. monocytogenes infection, the bacterial count in the liver and spleen was determined on day 5 of infection. As shown in Fig. 2(a), bacterial burden in the liver and spleen significantly increased in the Vδ1−/− mice compared to the wild-type mice. When a higher dose of L. monocytogenes (1/2 LD50 for the wild-type C57BL/6 mice) was inoculated, half of the Vδ1−/− mice died within 10 days after the infection while all of the wild-type mice survived (Fig. 2b). These results suggested that Vγ6/Vδ1+γδ T cells are important in innate protective immunity against L. monocytogenes infection.
Figure 2.
Exacerbated innate protective immunity against Listeria monocytogenes infection in the Vδ1−/− mice. (a) The wild type (WT) or Vδ1−/− mice were infected intraperitoneally (i.p.) with 5 × 104 colony-forming units (CFU) of L. monocytogenes, and bacterial counts in the liver and spleen were determined on day 5 after infection. Data that are representative of three independent experiments are shown. *P < 0·05 compared to WT mice. (b) The WT and Vδ1−/− mice were inoculated i.p. with 2·5 × 105 CFU L. monocytogenes, and survival rates of the mice were monitored for 2 weeks. The survival rates of the WT and Vδ1−/− mice were significantly different (P = 0·003). Two experiments showed nearly the same results, and representative data are demonstrated.
Formation of abscess-like lesions and increase of macrophage infiltration in the liver of the Vδ1−/− mice after L. monocytogenes infection
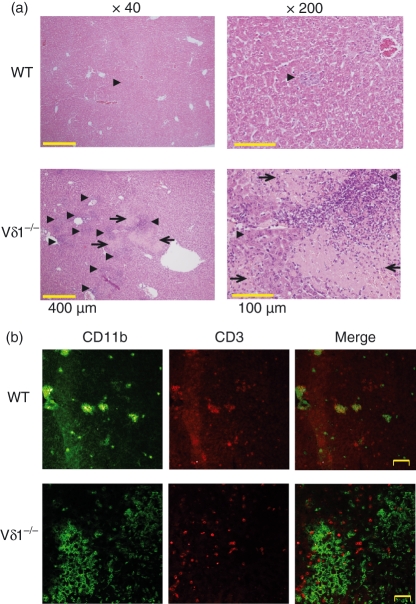
To analyse the mechanism of the Vγ6/Vδ1+γδ T-cell-mediated protection, we compared the liver histology of the Vδ1−/− mice and wild-type mice on day 5 of L. monocytogenes infection. The wild-type mice showed small granulomatous inflammatory lesions in sections with haematoxylin & eosin staining (arrow head in Fig. 3a upper panels), these consisted of colocalized CD11b+ cells and CD3+ cells in CF-LSM analysis (Fig. 3b, upper panels). In contrast, Vδ1−/− mice showed a higher number of inflammatory lesions (Fig. 3a, arrowheads in lower panels) with large, abscess-like lesions (Fig. 3a, arrows in lower panels) in the infected liver. Furthermore, the Vδ1−/− mice failed to show an organized granulomatous lesion with colocalization of CD11b+ and CD3+ cells in CF-LSM (Fig. 3b, lower panels).
Figure 3.
Histological examination of the liver of the wild-type (WT) and Vδ1−/− mice on day 5 of L. monocytogenes infection. (a) Haematoxylin & eosin staining of the liver sections from the WT and Vδ1−/− mice is demonstrated. There were only a few granulomatous lesions of inflammatory cell accumulation (arrowhead) in the WT mice (upper panels). In contrast, the liver sections from Vδ1−/− mice (lower panels) showed higher numbers of inflammatory cell-accumulated lesions (arrowheads), and large abscess-like lesions (arrows). (b) Confocal laser scanning microscopy analysis of the expression of CD11b and CD3 is shown on the liver sections from the WT and Vδ1−/− mice. In the WT mice, accumulated CD11b+ cells in the liver colocalized with CD3+ cells (upper panels) while colocalization of CD11b+ cells and CD3+ cells was hardly observed in the liver of the Vδ1−/− mice (lower panels).
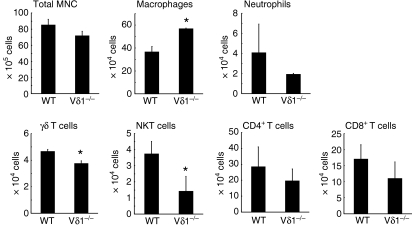
Next, the infiltration of inflammatory cells in the liver of the Vδ1−/− and wild-type mice was analysed 5 days after L. monocytogenes infection. Although the total liver mononuclear cell counts were not significantly different between the wild-type and Vδ1−/− mice, the number of macrophages was significantly higher in the Vδ1−/− mice compared to the wild-type mice (Fig. 4). The number of γδ T cells was slightly lower in the Vδ1−/− mice, which may be because of the lack of Vγ6/Vδ1+γδ T cells. The number of natural killer T cells was also lower in the Vδ1−/− mice. In contrast, the numbers of neutrophils and conventional CD4+ and CD8+ T cells in the livers of Vδ1−/− mice were similar to those in the livers of wild-type mice.
Figure 4.
The number of inflammatory cells in the livers of wild-type (WT) and Vδ1−/− mice on day 5 of Listeria monocytogenes infection. The ratios of macrophages, neutrophils and T-cell subpopulations were determined by flow cytometry and the absolute numbers of the cell populations were calculated as described in the Materials and methods section. *P < 0·05 compared to WT mice.
IFN-γ and IL-17A production by the Vγ6/Vδ1+γδ T cells in the liver of L. monocytogenes-infected mice
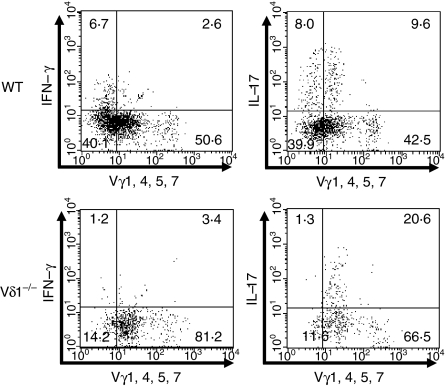
It is well established that proinflammatory cytokines have a pivotal role in regulation of the innate immune response against L. monocytogenes infection. Both IFN-γ and IL-17A are reported as proinflammatory cytokines produced by γδ T cells.2–5 Therefore, expression of the cytokines was analysed by FCM on the liver γδ T cells of the wild-type and Vδ1−/− mice after L. monocytogenes infection. When the γδ T cells of the wild-type mice were stained with the anti-Vγ mAb mixture and anti-cytokine mAb, expression of IL-17A and IFN-γ was detected not only in the anti-Vγ mAb mixture-positive fraction but also in the anti-Vγ mAb mixture-negative fraction represented by the Vγ6/Vδ1+γδ T cells (Fig. 5, upper panels). Interestingly, the anti-Vγ mAb mixture-negative γδ T cells contained more IFN-γ-producing γδ T cells compared to the anti-Vγ mAb mixture-positive γδ T cells. Analysis of the liver γδ T cells from L. monocytogenes-infected Vδ1−/− mice showed that IFN-γ expression by the γδ T cells decreased in the liver γδ T cells as the result of a lack of IFN-γ-producing Vγ6/Vδ1+γδ T cells in the anti-Vγ mixture-negative fraction (Fig. 5, lower panels). In contrast, expression of IL-17A was maintained in the liver γδ T cells of the Vδ1−/− mice because IL-17A production by the anti-Vγ mAb mixture-positive γδ T cells, especially Vγ4+γδ T cells (data not shown), increased in the mice. The result suggests that proinflammatory cytokines IFN-γ and IL-17A, especially IFN-γ, may participate in Vγ6/Vδ1+γδ T-cell-mediated regulation of innate immunity against L. monocytogenes infection.
Figure 5.
Expression of interleukin-17A (IL-17A) and interferon-γ (IFN-γ) by the γδ T cells induced in the livers of the wild-type (WT) and Vδ1−/− mice on day 5 of Listeria monocytogenes infection. The liver γδ T cells were enriched, and stimulated in vitro with phorbol 12-myrsitate 13-acetate and calcium ionophore for 4 hr in the presence of brefeldin A. The cells were stained with anti-TCR Cδ monoclonal antibody (mAb), anti-Vγ mAb mixture, and anti-IL-17A or anti-IFN-γ mAb, and were analysed by flow cytometry. The analysis gate was set on the TCR Cδ+ T cells in all panels. Experiments were repeated three to five times, and representative data are shown.
Discussion
It has been reported that γδ T cells have a pivotal role in innate protective immunity against L. monocytogenes infection in the liver.8–10 The γδ T cells induced by L. monocytogenes infection have been divided based on their Vγ and Vδ repertoires into Vγ1+, Vγ4+, and Vγ6/Vδ1+ T cells.11–15 The role of Vγ6/Vδ1+γδ T cells was analysed using mice deficient for both Vγ4 and Vγ6 genes; however, function of the Vγ6/Vδ1+γδ T cells could not be separated from that of Vγ4+γδ T cells in the mice.13 In the present report, the importance of the Vγ6/Vδ1+γδ T cells in the protective immunity against L. monocytogenes was demonstrated using Vδ1−/− mice, which selectively lack Vγ6/Vδ1+γδ T cells in the L. monocytogenes-infected liver.
Various stimuli induce Vγ6/Vδ1+γδ T cells in inflammatory sites. It has been reported that systemic infection of L. monocytogenes induces Vγ6/Vδ1+γδ T cells expressing invariant junctional sequences developed in the fetal thymus.14,15 The invariant Vγ6/Vδ1+γδ T cells are also induced in the peritoneal cavity of mice after i.p. inoculation of Escherichia coli.19,24 Furthermore, in a murine autoimmune orchitis model,25,26 infiltration of the invariant Vγ6/Vδ1+γδ T cells was detected in the lesions produced by the autoimmune inflammatory response in the testis. All the results suggest that Vγ6/Vδ1+γδ T cells are induced in the inflammatory site independent of the recognition of exogenous bacterial antigens. Consistent with these observations, Vγ6/Vδ1 soluble TCR-octamer stained murine cells such as keratinocytes and fibroblasts in the absence of exogenous antigen, indicating that the Vγ6/Vδ1 TCR is specific for self-surface molecules or molecular complexes.2,27 Although ligand specificity of the Vγ6/Vδ1+γδ T cells is still unclear, the observations suggest that the Vγ6/Vδ1+γδ T cells participate in immune surveillance in inflammatory sites through recognition of self antigen.
The Vγ6/Vδ1+γδ T cells may participate in innate immunity against infections through production of proinflammatory cytokines. Our data demonstrated that the Vγ6/Vδ1+γδ T cells produced proinflammatory cytokines IL-17A and IFN-γ. Interferon-γ is an important cytokine against L. monocytogenes infection at an innate immunity level.28,29 It is possible that formation of early granulomatous lesions in the L. monocytogenes-infected liver depends on the Vγ6/Vδ1+γδ T-cell-derived IFN-γ because the importance of the IFN-γ in granuloma formation is well established.29,30 Interleukin-17A produced by the γδ T cells also has a pivotal role in innate protection against L. monocytogenes (Hamada et al. unpublished observation). Production of IL-17A by the Vγ6/Vδ1+γδ T cells was also reported in E. coli infection.31 Therefore, both IL-17A and IFN-γ produced by the Vγ6/Vδ1+γδ T cells may be important in innate protective immunity against various pathogens. However, we estimate that IFN-γ production by the Vγ6/Vδ1+γδ T cells would be more important in Vγ6/Vδ1+γδ T-cell-mediated protection because IL-17A production of the γδ T cells was compensated by Vδ1-negative γδ T cells but infection was exacerbated in the Vδ1−/− mice. Further analysis is required to clarify the relative importance of IFN-γ compared to IL-17A in the Vγ6/Vδ1+γδ T-cell-mediated protection.
Although our data support a proinflammatory role of the Vγ6/Vδ1+γδ T cells, it is possible that the Vγ6/Vδ1+γδ T cells are also regulatory against inflammation. Histological analysis of the liver of L. monocytogenes-infected Vδ1−/− mice showed abscess-like lesions which were indistinguishable from those in the mice that were deficient for all γδ T cells,9,10 suggesting the possibility that the Vγ6/Vδ1+γδ T cells participate in innate immunity not only through the induction of inflammatory cells, but also through the suppression of an excessive inflammatory response that damages infected tissues. In agreement with this, depletion of γδ T cells accelerated the inflammatory response in the testis of mice with autoimmune orchitis with Vγ6/Vδ1+γδ T-cell induction in the autoimmune lesions.32 The result suggests a regulatory mechanism for Vγ6/Vδ1+γδ T cells against inflammation. Vγ6+γδ T cells were also shown to suppress Bacillus subtilis-induced pulmonary fibrosis through suppression of CD4+ and CD8+ T-cell responses.33 Therefore it is possible that the Vγ6/Vδ1+γδ T cells differentiate not only into proinflammatory (IFN-γ-producing or IL-17A-producing) T cells but also into anti-inflammatory T cells, and optimize the inflammatory response to eliminate pathogens and protect organs from tissue injury. Further research is required to clarify the anti-inflammatory function of the Vγ6/Vδ1+γδ T cells.
Acknowledgments
We thank Dr Pablo Pereira for kindly providing the 2.11 and F2.67 mAbs. This work was supported, in part, by the Programme of Founding Research Centres for Emerging and Reemerging Infectious Diseases from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan, a Grants-in-Aid for Scientific Research from the Japan Society for Promotion of Science (JSPS), and a grant from the Takeda Science Foundation.
References
- 1.Hayday AC. γδ T cells: a right time and right place for a conserved third way of protection. Annu Rev Immunol. 2000;18:975–1026. doi: 10.1146/annurev.immunol.18.1.975. [DOI] [PubMed] [Google Scholar]
- 2.O’Brien RL, Roark CL, Jin N, et al. γδ T cell receptor: functional correlation. Immunol Rev. 2007;215:77–88. doi: 10.1111/j.1600-065X.2006.00477.x. [DOI] [PubMed] [Google Scholar]
- 3.Ferrick DA, Schrenzel MD, Mulvania T, Hsieh B, Ferlin WG, Lepper H. Differential production of interferon-γ and interleukin-4 in response to Th1 and Th2-stimulating pathogens by γδ T cells in vivo. Nature. 1995;373:255–7. doi: 10.1038/373255a0. [DOI] [PubMed] [Google Scholar]
- 4.Umemura M, Yahagi A, Hamada S, et al. IL-17-mediated regulation of innate and acquired immune response against pulmonary Mycobacterium bovis bacille Calmette–Guérin infection. J Immunol. 2007;178:3786–96. doi: 10.4049/jimmunol.178.6.3786. [DOI] [PubMed] [Google Scholar]
- 5.Roark CL, French JD, Taylor MA, Bendele AM, Born WK, O'Brien RL. Exacerbation of collagen-induced arthritis by oligoclonal, IL-17-producing γδ T cells. J Immunol. 2007;179:5576–83. doi: 10.4049/jimmunol.179.8.5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakata M, Smyth MJ, Norihisa Y, Kawasaki A, Shinkai Y, Okumura K, Yagita H. Constitutive expression of pore-forming protein in peripheral blood γ/δ T cells: implication for their cytotoxic role in vivo. J Exp Med. 1990;172:1877–80. doi: 10.1084/jem.172.6.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andrew EM, Carding SR. Murine γδ T cells in infections: beneficial or deleterious? Microb Infect. 2005;7:529–36. doi: 10.1016/j.micinf.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 8.Hiromatsu K, Yoshikai Y, Matsuzaki G, Ohga S, Muramori K, Matsumoto K, Bluestone JA, Nomoto K. A protective role of γ/δ T cells in primary infection with Listeria monocytogenes in mice. J Exp Med. 1992;175:49–56. doi: 10.1084/jem.175.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mombaerts P, Arnordi J, Russ F, Tonegawa S, Kaufmann SHE. Different roles of αβ and γδ T cells in immunity against an intracellular bacterial pathogen. Nature. 1993;365:53–6. doi: 10.1038/365053a0. [DOI] [PubMed] [Google Scholar]
- 10.Fu Y-X, Roark CE, Kelly K, Drevets D, Campbell P, O'Brien R, Born W. Immune protection and control of inflammatory tissue necrosis by γδ T cells. J Immunol. 1994;153:3101–15. [PubMed] [Google Scholar]
- 11.Belles C, Kuhl AL, Donoghue AJ, Sano Y, O'Brien RL, Born W, Bottomly K, Carding SR. Bias in the γδ T-cell response to Listeria monocytogenes: Vδ6.3+ cells are a major component of the γδ T-cell response to Listeria monocytogenes. J Immunol. 1996;156:4280–9. [PubMed] [Google Scholar]
- 12.Nakamura T, Matsuzaki G, Nomoto K. The protective role of T-cell receptor Vγ1+ T cells in primary infection with Listeria monocytogenes. Immunology. 1999;96:29–34. doi: 10.1046/j.1365-2567.1999.00666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Brien RL, Yin X, Huber SA, Ikuta K, Born WK. Depletion of a γδ T-cell subset can increase host resistance to a bacterial infection. J Immunol. 2000;165:6472–9. doi: 10.4049/jimmunol.165.11.6472. [DOI] [PubMed] [Google Scholar]
- 14.Roark CE, Vollmer MK, Campbell PA, Born WK, O'Brien RL. Response of a γδ T cell receptor invariant subset during bacterial infection. J Immunol. 1996;156:2214–20. [PubMed] [Google Scholar]
- 15.Roark CL, Aydintug MK, Lewis J, et al. Subset-specific, uniform activation among Vγ6/Vδ1+γδ T cells elicited by inflammation. J Leukoc Biol. 2004;75:68–75. doi: 10.1189/jlb.0703326. [DOI] [PubMed] [Google Scholar]
- 16.Dalton JE, Pearson J, Scott P, Carding SR. The interaction of γδ T cells with activated macrophages is a property of the Vγ1 subset. J Immunol. 2003;171:6488–94. doi: 10.4049/jimmunol.171.12.6488. [DOI] [PubMed] [Google Scholar]
- 17.Hara H, Kishihara K, Matsuzaki G, Takimoto H, Tsukiyama T, Tigelaar RE, Nomoto K. Development of dendritic epidermal T cells with skewed diversity of γδ TCRs in Vδ1-deficient mice. J Immunol. 2000;165:3695–705. doi: 10.4049/jimmunol.165.7.3695. [DOI] [PubMed] [Google Scholar]
- 18.Watanabe H, Miyaji C, Seki S, Abo T. c-kit+ stem cells and thymocyte precursors in the liver of adult mice. J Exp Med. 1996;184:687–93. doi: 10.1084/jem.184.2.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsuzaki G, Takada H, Nomoto K. Escherichia coli infection induces only fetal thymus-derived γδ T cells at the infected site. Eur J Immunol. 1999;29:3877–86. doi: 10.1002/(SICI)1521-4141(199912)29:12<3877::AID-IMMU3877>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 20.Heilig JS, Tonegawa S. Diversity of murine gamma genes and expression in fetal and adult T lymphocytes. Nature. 1986;322:836–40. doi: 10.1038/322836a0. [DOI] [PubMed] [Google Scholar]
- 21.Perreira P, Gerber D, Huang SY, Tonegawa S. Ontogenic development and tissue distribution of Vγ1-expresing γ/δ T lymphocytes in normal mice. J Exp Med. 1995;182:1921–30. doi: 10.1084/jem.182.6.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pereira P, Hermitte V, Lembezat M-P, Boucontet L, Azura V, Grigoriadou K. Developmentally regulated and lineage-specific rearrangement of T cell receptor Vα/δ gene segments. Eur J Immunol. 2000;30:1988–97. doi: 10.1002/1521-4141(200007)30:7<1988::AID-IMMU1988>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 23.Ito K, Bonneville M, Takagaki Y, Nakanishi N, Kanagawa O, Krecko EG, Tonegawa S. Different γδ T-cell receptors are expressed on thymocytes at different stages of development. Proc Natl Acad Sci U S A. 1989;86:631–5. doi: 10.1073/pnas.86.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mokuno Y, Matsuguchi T, Takano M, et al. Expression of Toll-like receptor 2 on γδ T cells bearing invariant Vγ6/Vδ1 induced by Escherichia coli infection in mice. J Immunol. 2000;165:931–40. doi: 10.4049/jimmunol.165.2.931. [DOI] [PubMed] [Google Scholar]
- 25.Mukasa A, Lahn M, Pflum EK, Born W, O'Brien RL. Evidence that the same γδ T cells respond during infection-induced and autoimmune inflammation. J Immunol. 1997;159:5787–94. [PubMed] [Google Scholar]
- 26.Mukasa A, Born WK, O'Brien RL. Inflammation alone evokes the response of TCR-invariant mouse γδ T cell subset. J Immunol. 1999;162:4910–3. [PubMed] [Google Scholar]
- 27.Aydintug MK, Roark CL, Yin X, Wands JM, Born WK, O'Brien RL. Detection of cell surface ligands for the γδ TCR using soluble TCRs. J Immunol. 2004;172:4167–75. doi: 10.4049/jimmunol.172.7.4167. [DOI] [PubMed] [Google Scholar]
- 28.Huang S, Hendriks W, Althage A, et al. Immune response in mice that lack the interferon-γ receptor. Science. 1993;259:1742–5. doi: 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]
- 29.Dai WJ, Bartens W, Köhler G, Hufnagel M, Kopf M, Brombacher F. Impaired macrophage listericidal and cytokine activities are responsible for the rapid death of Listeria monocytogenes-infected IFN-γ receptor-deficient mice. J Immunol. 1997;158:5297–304. [PubMed] [Google Scholar]
- 30.Mielke ME, Peters C, Hahn H. Cytokines in the induction and expression of T-cell-mediated granuloma formation and protection in the murine model of listeriosis. Immunol Rev. 1997;158:79–93. doi: 10.1111/j.1600-065x.1997.tb00994.x. [DOI] [PubMed] [Google Scholar]
- 31.Shibata K, Yamada H, Hara H, Kishihara K, Yoshikai Y. Resident Vδ1+γδ T cells control early infiltration of neutrophils after Escherichia coli infection via IL-17 production. J Immunol. 2007;178:4466–72. doi: 10.4049/jimmunol.178.7.4466. [DOI] [PubMed] [Google Scholar]
- 32.Mukasa A, Hiromatsu K, Matsuzaki G, O'Brien R, Born W, Nomoto K. Bacterial infection of the testis leading to autoaggressive immunity triggers apparently opposed responses of αβ and γδ T cells. J Immunol. 1995;155:2047–56. [PubMed] [Google Scholar]
- 33.Simonian PL, Roark CL, del Valle FD, et al. Regulatory role of γδ T cells in the recruitment of CD4+ and CD8+ T cells to lung and subsequent pulmonary fibrosis. J Immunol. 2006;177:4436–43. doi: 10.4049/jimmunol.177.7.4436. [DOI] [PubMed] [Google Scholar]