Abstract
The purpose of this study was to examine the distribution of brevetoxin-3 administered to pregnant dams and to determine the extent of placental transport to fetuses. Twenty-nine pregnant CD-1 mice were administered 3H-brevetoxin-3 (~1.3 μCi/animal; ~2.8 μg compound/kg) by intratracheal instillation on one of gestational days 15–18. Groups of four or five dams were killed at selected times through 48 h post dosing. Four pregnant dams were administered 3H-brevetoxin-3 on gestational day 15 or 16 via osmotic minipump to provide continuous delivery of compound (~0.13 μCi, 7.5 ng compound/day) over a 72-h period after which the dams and fetuses were d. Brevetoxin-associated radioactivity was detected in placentas and fetuses within 0.5 h of intratracheal administration. Concentrations of brevetoxin equivalents in fetuses were approximately 0.3 ng/g throughout the 48-h post dosing, resulting in a calculated dose to fetuses of 19 ng/g-h. Following brevetoxin infusion, concentration of brevetoxin equivalents in fetuses was 0.1 ng/g, lower than that present in most maternal tissues. Results demonstrated placental transport of brevetoxin or its metabolites following maternal acute exposure and repeated low-dose exposure. The consequences of these findings for pregnant women exposed to brevetoxins by inhalation or ingestion remain to be determined.
Keywords: Brevetoxin-3, placental transport, intratracheal instillation, mice
1. Introduction
Brevetoxins are naturally occurring environmental contaminants produced by the several species of dinoflagellates, most notably, Karenia brevis (K. brevis). K. brevis is the organism responsible for the red tides occurring primarily in the Gulf of Mexico and sporadically along the Atlantic Coast of Florida. Brevetoxins are lipid soluble neurotoxins. In humans, exposure to brevetoxins produces two pronounced effects, depending on the route of exposures. With ingestion of brevetoxin-contaminated shellfish, neurotoxic shellfish poisoning (NSP) occurs with mild gastroenteritis and neurological symptoms. Inhalation of brevetoxins aerosolized by wind and surf produces irritation of mucous membranes and airways (Baden et al., 1982; Kirpatrick et al., 2004). Brevetoxin-induced bronchoconstriction is well documented in humans following inhalation in occupational and recreational settings (Backer et al., 2003, 2005; Fleming et al., 2005a,b) and in sheep in laboratory experiments (Abraham et al., 2005a,b).
Experiments in laboratory rats and mice have shown that brevetoxins are rapidly absorbed and widely distributed among tissues following a single administration by ingestion (Cattet and Geraci, 1993), intravenous injection (Poli et al., 1990a,b), and intratracheal instillation (Benson et al., 1999; Tibbetts et al., 2006). As expected, relative concentrations of brevetoxin in tissues vary with route of administration and time after dosing. For example, concentrations of brevetoxin equivalents were highest in liver following oral administration in rats (Cattet and Gerachi, 1993), and highest in lung following intratracheal instillation (Benson et al., 1999). Concentrations of brevetoxin equivalents in brain were among the lowest of all tissues examined following both oral and intratracheal administration. Regardless of the exposure route, muscle, GI tract, and liver contained the largest percentages of the administered dose. Muscle appears to serve as a sink for brevetoxins, while liver and GI tract are major organs of metabolism and excretion. Few differences between rats and mice exist in the distribution of intratracheally instilled brevetoxin; however, clearance rates differ somewhat between the species (Benson et al., 1999; Tibbetts et al., 2006).
Brevetoxin-3 undergoes metabolism in rodents (Poli et al., 1990a,b and Benson et al., unpublished observations). However these metabolites remain to be identified. In vitro studies indicated brevetoxins 1 and 2 are metabolized by rat hepatocytes and liver microsomal enzymes, suggesting P450 mediated metabolism (Wang et al., 2005). Studies by Poli et al. (2000) evaluating metabolites of brevetoxin in urine of individuals with NSP and in brevetoxin-contaminated shellfish suggest that brevetoxin metabolites may also be toxic. Brevetoxin is eliminated from the body in both feces and urine, with 78% (Cattet and Geraci, 1993) to greater than 90% (Poli et al., 1990a,b) being eliminated within 7 days of exposure.
In addition to being potent neurotoxicants, recent data also suggest that brevetoxin exposure may also adversely affect the immune system. Kirkpatrick et al. (2006) have reported increased frequency of emergency room visits for respiratory related illnesses, including pneumonia, during Florida red tide events. Brevetoxin-induced inhibition of antibody production has been found in rats (Benson et al., 2004, 2005). In addition, Sayer et al. (2005) reported DNA damage in human lymphocytes exposed to nanomolar concentrations of brevetoxins in vitro. The latter findings of genotoxicity have implications beyond adverse effects to the immune system. The wide tissue distribution of brevetoxin following exposure suggests the feasibility of placental transport, with possible binding to targets for toxicity in the embryo or fetus.
Placental transfer of environmental contaminants in humans and laboratory animals is well documented for several classes of lipophilic environmental pollutants, including halogenated organic compounds (Guvenius et al., 2003; Mazdai et al., 2003; Meerts et al., 2002; Sinjari and Darnerud, 1998; Roman et al., 1998) and polycyclic aromatic hydrocarbons (Perera et al., 1999, and references cited therein). Further evidence indicates that fetuses are more susceptible than adults to a variety of environmental toxicants (Perera et al., 1999). The developing nervous system is a particularly sensitive target for environmental toxicants (Rodier, 1995; Needleman, 1995). In addition, organogenesis and development of the immune system occurs during the prenatal, and to a lesser extent, the early postnatal periods of mammalian development. Studies suggest that the fetus may be more prone to genetic damage caused by certain agents because of differences in concentrations of microsomal metabolizing enzymes, DNA repair enzymes, or in extent of body fat and muscle that can serve as a sink for lipid soluble toxicants.
To date, no studies have addressed the placental transfer or developmental toxicity of brevetoxins in mammals. The purpose of this study was to examine the tissue distribution of brevetoxin-3 administered to pregnant dams and to determine the existence and extent of placental transport of the parent compound or its metabolites to fetuses following acute and repeated exposure. Brevetoxin-3 was chosen for these studies because it is a major component of brevetoxin-containing aerosols along red tide affected beaches (Cheng et al., 2005). Further, it is a potent neurotoxin in vitro (Baden, 1989) and suppresses humoral immunity in rats following repeated inhalation (Benson et al., 2004, 2005).
2. Materials and methods
2.1. Chemicals
Brevetoxin-3 was isolated and purified from the Wilson clone of K. brevis at the Center for Marine Science at the University of North Carolina at Wilmington, NC. 3H-Brevetoxin-3 (C-42; 15.5 Ci/mM) was also prepared by the Center for Marine Science, using the method described by Poli and coworkers (1986). The tritium label is covalently attached at C-42 and is nonexchangeable. The radiochemical purity of the 3H-brevetoxin-3, as determined by high-performance liquid chromatography (HPLC), exceeded 95%. The dosing solution had a specific activity of 7.94 Ci/mM (10 μCi/mL; 1.13 μg brevetoxin-3/mL dosing solution; 8.85 μCi/μg).
Acetone (HPLC grade), methanol (biotech grade), and hexane (HPLC grade) were purchased from Fischer Scientific (Hampton, NJ).
2.2. Animals
Male and female CD-1 mice were purchased from Charles River Laboratories (Wilmington, MA). The mice were 10–11 weeks old when received. Animals were housed in shoebox cages with hardwood chip bedding and filter tops. The animal rooms were maintained at 20–22 °C with a relative humidity of 20–50% and a 12-h light cycle beginning at 0600. Food (Harlan Teklad, Madison, WI) and water were provided ad libitum. All housing was in compliance with the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996). The study protocol was reviewed and approved by the Lovelace Respiratory Research Institute’s Animal Care and Use Committee. For breeding, one male and one female were paired on 3–4 consecutive nights and females were checked for sperm plugs the following mornings. The morning when a plug was present was counted as gestational day 1. Pregnant females were housed separately.
3. Experimental design
3.1. Intratracheal instillation study
Twenty-nine pregnant mice were administered 3H-brevetoxin-3 (1.3 μCi, 0.14 μg/animal, ~2.8 μg brevetoxin-3/kg body weight) by intratracheal instillation on one of gestational days 15–18. The mice were anesthetized using 5% isoflurane in a 50:50 mixture of oxygen and nitrous oxide. A catheter was placed transorally into the trachea for administration of the dose in 0.1 mL saline containing 0.1% Tween 80. Aliquots (0.1 mL) of dosing solution were collected for estimation of the initial doses to the instilled mice (initial body burden).
Dosed mice were killed by intraperitoneal injection of Euthasol® (468 mg/kg sodium pentobarbital; 60 mg/kg phenytoin) at 0.5, 1, 4, 8, 16, 24, and 48 h post-instillation. At sacrifice, maternal blood, liver, kidneys, spleen, lung, brain, ovaries, GI tract with contents, pelt, uterus, placentas (pooled), and fetuses (pooled) were removed and weighed. Fetuses were euthanized by decapitation. All tissue samples were immediately processed for radiochemical analysis. In one instance, milk was obtained from the stomachs from pups born prior to a designated 48-h sacrifice. The dam and pups were killed and tissues processed as for the other mice.
3.2. Repeated exposure by osmotic pump
Four pregnant mice were surgically implanted with Alzet® micro-osmotic pumps (DURECT Corp., Cupertino, CA) while under isoflurane anesthesia on gestational days 15–16. Target daily doses were 1 μCi 3H-brevetoxin-3/day. The daily dose was intended to be equivalent to that provided once as a bolus dose by intratracheal instillation. Continuous compound release by the pump was intended to simulate slow continuous absorption as may occur following inhalation over a prolonged period, and to assess whether tissue accumulation of compound may occur with continuous exposure over several days. Actual daily doses, determined by in vitro experimentation, were 0.13 μCi 3H-brevetoxin (containing approximately 14 ng brevetoxin-3). The animals exposed to 3H-brevetoxin-3 were killed and processed as described for tracheally instilled animals.
3.3. Radiochemical analysis
The tissue samples were digested in toto using a 35% solution of tetraethylammonium hydroxide (~1 mL/g tissue; Sachem, Inc., Austin, TX), and the weight of the each digestate was recorded. Weighed aliquots of digestate (~1 g) were neutralized with concentrated hydrochloric acid (VWR International, West Chester, PA), decolorized with 30% hydrogen peroxide (ACS grade; Fisher Scientific, Hampton, NH), and mixed with Ultima Gold XR® scintillation cocktail (Packard Instrument Co., Meriden, CT) for quantitation of tritium activity by liquid scintillation counting. Aliquot counts were then used to calculate the total activity for the corresponding digestate.
3.4. Measurement of parent brevetoxin-3 in fetal tissues
In order to determine if parent brevetoxin-3 was present in fetuses following maternal exposure for 72 h, eight pregnant dams were surgically implanted with osmotic pumps as described above. Four dams received daily brevetxoin-3 doses of approximately 0.3 μg/kg/day. The remaining four dams received vehicle alone (phosphate buffered saline containing 0.1% Tween 80). Fetuses from dams exposed to vehicle or to brevetoxin-3 were pooled within their groups, homogenized, and subjected to solvent extractions using previously described methods (Naar et al., 2002). Briefly, pooled fetuses were homogenized in equal volumes of phosphate buffered saline. Nine, 1-g aliquots of each homogenate were extracted twice with 2 mL acetone. The acetone extracts were dried under nitrogen, redissolved in 80% methanol, and defatted by extraction with n-hexane. The defatted extracts were dried under nitrogen and redissolved in 25% methanol for purification on a C-18 separation column (Alltech, Deerfield, IL). The purified fractions were analyzed by liquid chromatography/mass spectrometry (LC/MS) for brevetoxin-3. Aliquots of control fetal homogenates were spiked with known quantities of brevetoxin-3 and subjected to the same extraction procedures. These samples were used to determine the efficiency of the extraction procedure and as positive controls for the LC/MS analyses.
Liquid chromatographic (LC) separation was conducted with a Shimadzu LC-10 ADVP fitted with a C18 “Aqua” column (3 micron, 125 A, 75 × 200 mm; Phenomenex #003-4311-B0, Torrance, CA) and C18 guard column. The column temperature was set at 32 °C (Eppendorf TC-50, Fisher Scientific). The flow rate was 150–200 μL/min. The injection volume was 50 μL with a 100 μL overfill (auto-injector). The mobile phases for chromatographic separation were purified water (solvent A) and methanol:1 mM ammonium acetate (solvent B) that were operated on the following time cycle: 20% B for 0–3 min, 90% B for 3–10 min, 20% B for 10–13 min.
Triple quadrupole mass spectrometry (MS3) was performed using an Applied Biosystems (Foster City, CA) 365 instrument. The data station was Analyst Version 1.3.1 (Agilent Technologies, Palo Alto, CA). Analytes were ionized using a heated nebulizer ionization source. The instrument was run in multiple response monitor mode and used the parent/daughter ion pairs of 897.5/725.4 to identify and quantify brevetoxin-3 components.
Quality control measures for each set of samples included analysis of unexposed tissue extracts as “blanks” to assess the potential for background contamination. Fetal homogenates were spiked with 250 ng/g and 500 ng/g tissue prior to extraction and analysis. The extraction efficiencies (mean ± SD; n =3) for fetal tissue spiked with 250 ng/g tissue and 500 ng/g were 63.6 ± 3.3 and 52.6 ± 1.4 percent, respectively.
3.5 Statistical analyses
Means and standard deviations of brevetoxin-3 equivalents in tissues were calculated using Microsoft Excel® (Redmond, WA) software. The kinetics of brevetoxin-equivalents elimination for each tissue were modeled with standard pharmacokinetic models using TableCurve 2D® software (SPSS Science, Chicago, IL). Estimates of total dose to tissue, calculated by integrating the area under the elimination curve from 0.5 h–48 h for each tissue, were also obtained using TableCurve 2®. Doses are expressed as nanogram brevetoxin-3 equivalents-h/g of tissue.
4. Results
4.1. Distribution of brevetoxin equivalents
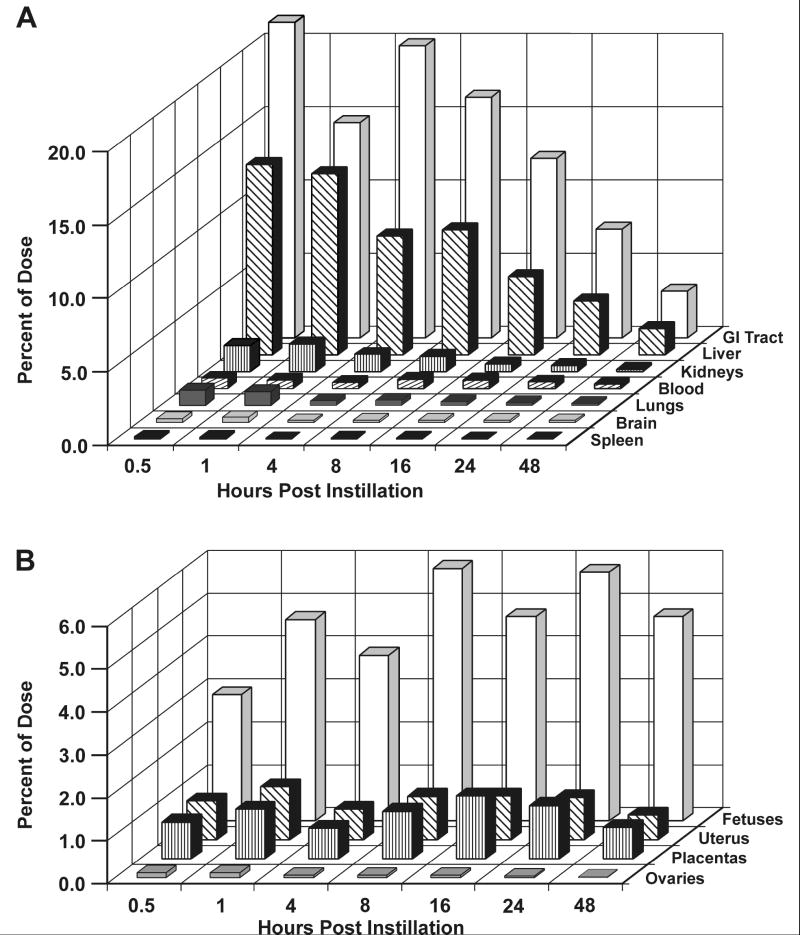
Intratracheally instilled brevetoxin-3 distributed rapidly throughout the pregnant dam, with the greatest percentages of brevetoxin-associated 3H-activity distributing in carcass (muscle), the tissue sinks for brevetoxin, as well as to the GI tract and liver, the primary organs of metabolism and excretion (Figure 1a). Following these tissues, the fetuses contained the next highest percentage of the administered dose with almost 3% present 0.5 h after dosing (Figure 1b). At 48 h post dosing, fetuses contained the highest percentage of brevetoxin-associated activity (4.76%) of all tissues examined, while approximately 1% of the administered activity was associated with placentas and uterus.
Figure 1.
a. Percentages of the administered brevetoxin dose present in selected maternal tissues up to 48 hours post dosing. Results are the means of 4 values except for all tissues at the 24 hour time point, where n = 5.
b. Percentages of administered brevetoxin dose in maternal blood, reproductive tissues and fetuses as a function of time after dosing. Results are the means of 4 values except for all tissues at the 24 hour time point, where n = 5 and placentas at 48 hr where n = 2.
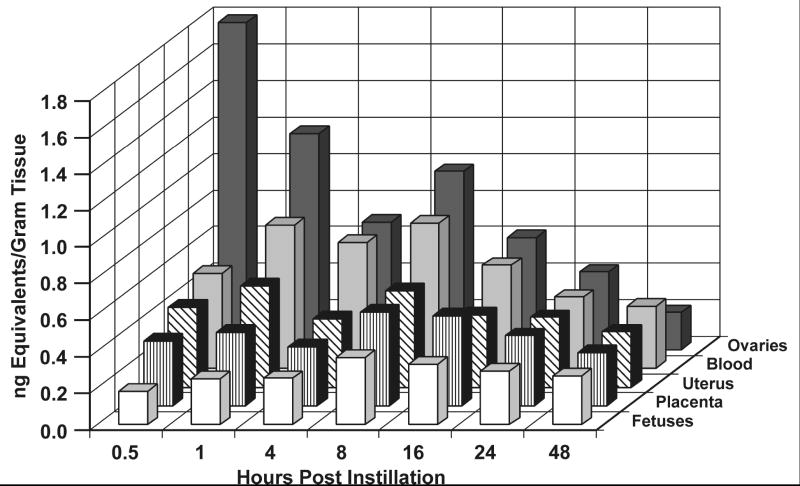
Initial concentrations of brevetoxin equivalents were highest in GI tract, kidney, liver, and lungs (Table 1). Transient increases in concentrations in kidney and GI tract between 1 and 8 h is likely related to clearance of parent compound or metabolites via urine and feces. Interestingly, concentrations in ovaries were greater than in blood, brain, spleen, uterus, placentas, and fetus through 24 h post dosing. While group mean concentrations of brevetoxin-associated activity in uterus, placentas, and fetuses were generally less than 0.5 ng/g tissue throughout the 48-h period post dosing, concentrations in these tissues remained fairly constant over time (Table 1, Figure 2). For example, concentrations of brevetoxin equivalents in carcass, GI tract, liver, lungs, ovaries, and spleen at 48 h were 25% or less than those present 0.5 h post dosing. Forty-eight-hour concentrations in blood, brain, and uterus were 50% or more of the 0.5-h concentration. By contrast, the 48-h concentration in fetuses was 150% of that at 0.5 h and similar to that present 1 to 24 h post dosing.
Table 1.
Concentrations of brevetoxin equivalents in dams administered 3H-brevetoxin-3 by intratracheal instillation and toxicokinetic parametersa
| Ng/g tissue
|
Limited toxicokinetic parameters | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Hours post dosing | ||||||||||
| 0.5 | 1 | 4 | 8 | 16 | 24b | 48 | t1/2(h) | Fixed Fraction | Dose to Tissue (ng/g-h) | |
| Blood | 0.52 ± 0.25 | 0.78 ± 0.38 | 0.69 ± 0.53 | 0.79 ± 0.44 | 0.56 ± 0.30 | 0.39 ± 0.29 | 0.34 ± 0.12 | 39.2 | 23.3 | |
| Brain | 0.43 ± 0.27 | 0.53 ± 0.12 | 0.27 ± 0.10 | 0.32 ± 0.09 | 0.29 ± 0.02 | 0.27 ± 0.08 | 0.23 ± 0.09 | 1.7 | 0.49 | 13.4 |
| Carcass | 1.36 ± 0.69 | 2.11 ± 0.36 | 0.83 ± 0.31 | 1.15 ± 0.78 | 0.47 ± 0.05 | 0.37 ± 0.05 | 0.22 ± 0.09 | 6.8 | 0.13 | 24.8 |
| GI tract and contents | 2.75 ± 2.51 | 1.80 ± 0.26 | 2.65 ± 0.11 | 2.39 ± 0.18 | 1.42 ± 0.46 | 0.96 ± 0.30 | 0.43 ± 0.23 | 14.1 | 57.4 | |
| Kidneys | 2.89 ± 1.84 | 4.14 ± 1.56 | 2.10 ± 0.62 | 1.87 ± 0.40 | 0.91 ± 0.19 | 0.68 ± 0.25 | 0.30 ± 0.13 | 6.4 | 0.10 | 45.3 |
| Liver | 4.25 ± 2.76 | 3.45 ± 0.48 | 2.47 ± 0.95 | 2.82 ± 0.26 | 1.40 ± 0.43 | 1.01 ± 0.22 | 0.54 ± 0.25 | 9.0 | 0.12 | 64.5 |
| Lungs | 2.98 ± 1.95 | 1.95 ± 0.77 | 1.09 ± 0.60 | 1.37 ± 0.30 | 0.77 ± 0.35 | 0.63 ± 0.29 | 0.46 ± 0.20 | 9.6 | 30.7 | |
| Ovaries | 1.79 ± 1.80 | 1.18 ± 0.22 | 0.69 ± 0.28 | 0.97 ± 0.34 | 0.61 ± 0.18 | 0.42 ± 0.29 | 0.20 ± 0.14 | 20.2 | 24.2 | |
| Pelt | 0.77 ± 0.32 | 0.96 ± 0.1 | 0.52 ± 0.20 | 0.65 ± 0.11 | 0.33 ± 0.13 | 0.35 ± 0.06 | 0.28 ± 0.14 | 6.2 | 0.33 | 18.6 |
| Spleen | 0.97 ± 0.84 | 0.80 ± 0.2 | 0.48 ± 0.16 | 0.55 ± 0.16 | 0.40 ± 0.07 | 0.32 ± 0.13 | 0.24 ± 0.09 | 16.9 | 16.6 | |
| Uterus | 0.44 ± 0.32 | 0.55 ± 0.08 | 0.37 ± 0.15 | 0.52 ± 0.06 | 0.40 ± 0.10 | 0.38 ± 0.07 | 0.30 ± 0.12 | 71.4 | 18.2 | |
| Placentas | 0.35 ± 0.25 | 0.40 ± 0.13 | 0.32 ± 0.12 | 0.51 ± 0.17 | 0.49 ± 0.07 | 0.38 ± 0.11 | 0.17, 0.41c | 43.6 | 19.4 | |
| Fetuses | 0.18 ± 0.13 | 0.25 ± 0.06 | 0.25 ± 0.14 | 0.37 ± 0.05 | 0.33 ± 0.03 | 0.29 ± 0.04 | 0.27 ± 0.09 | 83.6 | 14.1 | |
Mean ± SD, n = 4 unless otherwise noted.
n = 5.
n = 2.
Figure 2.
Concentrations of brevetoxin equivalents present in maternal blood, reproductive tissues and fetuses as a function of time after dosing. Results are the means of 4 values except for all tissues at the 24 hour time point, where n = 5 and placentas at 48 hr where n = 2.
Because of the short time frame of the evaluations, only limited toxicokinetic evaluations could be performed. Tissue clearance data were fit using single-component negative-exponential functions. Clearance halftimes for brevetoxin equivalents were greatest for fetuses, uterus, and placentas. The area under the curve evaluations from 0.5–48 h, however, show dose to fetuses of approximately 14 ng/g-h, the second lowest dose next to brain, of any tissue examined.
The concentration of brevetoxin-associated activity in milk obtained from the stomachs of pups born before the designated 48-h sacrifice was 0.26 ng/g milk, approximately half the concentration present in maternal blood at the time of sacrifice (0.43 ng/g).
Concentrations of brevetoxin equivalents in maternal tissues and fetuses from dams administered brevetoxin for 72 h beginning at gestational days 15 or 16 are summarized in Table 2. Blood, GI tract, kidneys, and liver contained the highest concentrations of brevetoxin equivalents. Concentrations in carcass, pelt, lungs, ovaries, spleen, uterus, and placentas were very similar, ranging from 0.14–0.18 ng/g tissue. Fetus and brain had the lowest concentrations, 0.10 and 0.7 ng/g, respectively.
Table 2.
Concentrations of brevetoxin equivalents in maternal and fetal tissue following 72 h of continuous administration by osmotic pumpa
| Tissue | ng/g tissue |
|---|---|
| Blood | 0.28 ± 0.23 |
| Brain | 0.07 ± 0.02 |
| Carcass | 0.13 ± 0.007 |
| GI tract and contents | 0.39 ± 0.10 |
| Kidneys | 0.24 ± 0.06 |
| Liver | 0.36 ± 0.07 |
| Lungs | 0.15 ± 0.02 |
| Ovaries | 0.17 ± 0.02 |
| Pelt | 0.16 ± 0.03 |
| Spleen | 0.14 ± 0.02 |
| Uterus | 0.15 ± 0.04 |
| Placentas | 0.18 ± 0.01 |
| Fetuses | 0.10 ± 0.02 |
Mean ± SD, n = 4.
4.2. Quantitation of parent brevetoxin-3 in fetuses
Parent brevetoxin-3 was not detected by mass spectrometry in extracts of pups from dams administered brevetoxin-3 by osmotic minipumps. The limit of quantitation for brevetoxin-3 was 1 ng/g tissue.
5. Discussion
The purpose of this study was to examine the uptake and tissue distribution of brevetoxin-3 administered to pregnant dams and to determine the existence and extent of placental transport to fetuses following a single intratracheal instillation and after 72 h of infusion by osmotic pump.
As has been reported for male mice and rats administered 3H-brevetoxin-3 by intratracheal instillation, the organs in pregnant dams with the highest percentages of the administered dose included the GI tract (with contents), carcass (primarily muscle and fat) and liver (Benson et al., 1999; Tibbetts et al., 2006). Further, as was found for adult male rats and mice, percentages of the initial administered dose distributing to potential target organs for toxicity, including brain and spleen, were less than 1%. Distribution of brevetoxin equivalents to ovaries of pregnant dams and testes of male mice (Tibbetts, et al., 2006) suggests exposure to germ cell populations to the potential genotoxic effects of brevetoxin (Sayer et al., 2005).
This study is the first to identify placental transport of brevetoxin. Clearance of brevetoxin equivalents from the fetuses was relatively slow following intratracheal instillation compared to other tissues, suggesting continuous redistribution of material to the fetus from other tissues. Slow absorption of brevetoxin by pregnant dams from the osmotic pumps, simulating absorption expected to occur following prolonged inhalation exposure, also resulted in distribution of brevetoxin equivalents to fetuses. Concentrations of brevetoxin equivalents in fetuses were slightly lower than concentrations in maternal tissues following 72 h of continuous exposure, but this is likely a consequence of total dose administered over the three days was very much less by minipump than by intratracheal instillation.
The presence of brevetoxin equivalents in fetuses and milk coupled with in vivo and in vitro evidence indicates that brevetoxins may be immunotoxic, suggesting the potential for adverse consequences resulting from pre- and perinatal exposure to brevetoxins. The perinatal period is a time of high sensitivity to toxicants, including immunotoxicants and neurotoxicants that cross the placenta or enter the neonate via lactation.
Parent brevetoxin-3 was not detected in fetal tissue by mass spectrometry. This is likely because the concentration of brevetoxin-3 was below the limit of detection of the method, but does not preclude the presence of brevetoxin metabolites in fetus. Although brevetoxin-3 is known to undergo mammalian metabolism (Poli et al., 1990a,b, 2000), the structural identity of mammalian metabolites of brevetoxin-3 and their toxicities have not been determined.
Placental transport of brevetoxin from mother to fetus is possible through at least two routes. The first is by diffusion across the placental membrane. Second, transporters, including organic anion or peptide transporters in placenta, may also participate in movement of toxicants across the placenta into fetal circulation (Unadkat et al., 2004; You, 2004) and rat placenta (Leazer and Klaassen, 2003). The mechanism of brevetoxin transport remains to be identified, but is likely to be a result of diffusion due to the high lipophilicity of the compound.
Among amphibians, K. brevis exposure has proven toxic to finfish larvae but not eggs (Riley et al., 1989) or sea urchin embryos (Moon and Morrill, 1976). Recently, Kimm-Brinson and Ramsdell (2001) reported neurotoxicity and developmental toxicity among Medaka fish embryos exposed to parts per million concentrations of brevetoxin-1 by microinjection. But as with any toxicant, effect is a function of dose. Measured brevetoxin concentrations in marine aerosols have generally been less than 25 ng/m3 and particle size distributions range from 6–12 μm mass median aerodynamic diameter, favoring deposition in the human upper respiratory tract (Cheng et al., 2005). Based on these data, a dose rate of approximately 1.2 ng/h per 1 ng brevetoxin/m3 air concentration has been derived (Cheng et al., 2005). Therefore, a pregnant woman might absorb up to 5 ng brevetoxin during a 4-h visit to a red tide-affected beach. Assuming a 60-kg woman, the dose would be approximately 84 pg/kg body weight. With complete systemic absorption and fairly equal distribution throughout the body tissues (based on results following infusion of brevetoxin via minipump), the estimated maternal and fetal tissue concentration would be quite low, 84 fg of brevetoxin or brevetoxin metabolites/g tissue.
The implications of fetal exposure to brevetoxins remains to be determined. As yet, it is not known if parent compounds or brevetoxin metabolites reach the fetus, whether metabolism occurs within the fetus, or what the relative toxicity of these metabolites is compared to parent compounds. Studies addressing the potential developmental toxicity of inhaled brevetoxins are planned.
Acknowledgments
This research was supported by a grant from the Florida Department of Health and by the National Institutes of Health, contract # P01 ES10594. The authors thank Ms. Sonia Lopez for excellent technical assistance, Dean Kracko for mass spectroscopy, and Ms. Barbara Elswick McCombie for many helpful discussions on this project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham WM, Bourdelais AJ, Sabater JR, Ahmed A, Lee TA, Serebriakov I, Baden DG. Airway responses to aerosolized brevetoxins in an animal model of asthma. Am J Respir Crit Care Med. 2005a;171:26–34. doi: 10.1164/rccm.200406-735OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham WM, Bourdelais AJ, Ahmed A, Serebriakov I, Baden DG. Effects of inhaled brevetoxins in allergic airways: Toxin-allergen interactions and pharmacologic intervention. Environ Health Perspect. 2005b;113(5):632–637. doi: 10.1289/ehp.7498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backer LC, Fleming LE, Rowan A, Cheng YS, Benson J, Pierce RH, Zaias J, Bean J, Bossart GD, Johnson D, Quimbo R, Baden DG. Recreational exposure to aerosolized brevetoxins during Florida red tide events. Harmful Algae. 2003;2:19–28. [Google Scholar]
- Backer LC, Kirkpatrick B, Fleming LE, Cheng YS, Pierce R, Bean JA, Clark R, Johnson D, Wanner A, Tamer R, Zhou Y, Baden D. Occupational exposure to aerosolized brevetoxins during Florida red tide events: Effects on a healthy worker population. Environ Health Perspect. 2005;113(5):644–649. doi: 10.1289/ehp.7502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baden DG, Mende JJ, Bikhazi G, Leung I. Bronchoconstriction caused by Florida red tide toxins. Toxicon. 1982;20:929–932. doi: 10.1016/0041-0101(82)90081-2. [DOI] [PubMed] [Google Scholar]
- Baden DG. Biochemistry of the brevetoxins: Potent activators of voltage-sensitive sodium channels. FASEB J. 1989;3:1807–1817. [Google Scholar]
- Benson JM, Tischler DL, Baden DG. Uptake, tissue distribution, and excretion of brevetoxin 3 administered to rats by intratracheal instillation. J Toxicol Environ Health A. 1999;56:345–355. doi: 10.1080/009841099157656. [DOI] [PubMed] [Google Scholar]
- Benson J, Hahn F, March T, McDonald J, Sopori M, Seagrave J, Gomez A, Bourdelais A, Naar J, Zaias J, Bossart G, Baden D. Inhalation toxicity of brevetoxin 3 in rats exposed for 5 days. J Toxicol Environ Health A. 2004;67(18):1443–1456. doi: 10.1080/15287390490483809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson JM, Hahn FF, March TH, McDonald JD, Gomez AP, Sopori ML, Bourdelais AJ, Naar J, Zaias J, Bossart GD, Baden DG. Inhalation toxicity of brevetoxin 3 in rats exposed for twenty-two days. Environ Health Perspect. 2005;113:626–631. doi: 10.1289/ehp.7497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattet M, Geraci JR. Distribution and elimination of ingested brevetoxin (PbTx3) in rats. Toxicon. 1993;31:1483–1486. doi: 10.1016/0041-0101(93)90214-4. [DOI] [PubMed] [Google Scholar]
- Cheng YS, Zhou Y, Irvin CM, Pierce RH, Naar J, Backer LC, Fleming LE, Kirkpatrick B, Baden DG. Characterization of marine aerosol for assessment of human exposure to brevetoxins. Environ Health Perspect. 2005;113:638–643. doi: 10.1289/ehp.7496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming LE, Backer LC, Baden DG. Overview of aerosolized Florida red tide toxins: Exposures and effects. Environ Health Perspect. 2005a;113(5):618–620. doi: 10.1289/ehp.7501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming LE, Kirkpatrick B, Backer LC, Bean JA, Wanner A, Dalpra D, Tamer R, Zaias J, Cheng YS, Pierce R, Naar J, Abraham W, Clark R, Zhou Y, Henry MS, Johnson D, Van de Bogart G, Bossart GD, Harrington M, Baden DG. Initial evaluation of the effects of aerosolized Florida red tide toxins (brevetoxins) in persons with asthma. Environ Health Perspect. 2005b;113(5):650–657. doi: 10.1289/ehp.7500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guvenius DM, Aronsson A, Ekman-Ordeberg G, Bergman A, Noren K. Human prenatal and postnatal exposure to polybrominated diphenyl ethers, polychlorinated biphenyls, polychlorobiphenylols, and pentachlorophenol. Environ Health Perspect. 2003;11:1235–1241. doi: 10.1289/ehp.5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holladay SD, Luster MI. Developmental immunotoxicology. In: Kimmel C, Buelke SJ, editors. Developmental Toxicology. 2. Raven Press; New York: 1994. pp. 93–118. [Google Scholar]
- Holladay SD, Smialowicz RJ. Development of the murine and human immune system: Differential effects of immunotoxicants depend on time of exposure. Environ Health Perspect. 2000;108(Suppl 3):463–473. doi: 10.1289/ehp.00108s3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimm-Brinson KL, Ramsdell JS. The red tide toxin, brevetoxin, induces embryo toxicity and developmental abnormalities. Environ Health Perspect. 2001;109:377–381. doi: 10.1289/ehp.01109377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick B, Fleming LE, Squicciarini D, Backer LC, Clark R, Abraham W, Benson J, Cheng Y-S, Johnson D, Pierce R, Zaias JK, Bosart GD, Baden DG. Literature review of Florida red tide: Implications for human health effects. Harmful Algae. 2004;3:99–115. doi: 10.1016/j.hal.2003.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick B, Fleming LE, Backer LC, Bean JA, Tamer R, Kirkpatrick G, Kane T, Wanner A, Dalpra D, Reich A, Baden DG. Environmental exposures to Florida red tides: Effects on emergency room respiratory diagnoses admissions. Harmful Algae. 2006 doi: 10.1016/j.hal.2005.09.004. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leazer TM, Klaassen CD. The presence of xenobiotic transporters in rat placenta. Drug Metab Dispos. 2003;31(2):153–167. doi: 10.1124/dmd.31.2.153. [DOI] [PubMed] [Google Scholar]
- Mazdai A, Dodder NG, Abernathy MP, Hites RA, Bigsby RM. Polybrominated diphenyl ethers in maternal and fetal blood samples. Environ Health Perspect. 2003;111:1249–1252. doi: 10.1289/ehp.6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerts IA, Assink Y, Cenijin PH, Van Den Berg JH, Weijers BM, Bergman A, Koeman JH, Brouwer A. Placental transfer of a hydroxylated polychlorinated biphenyl and effects on fetal and maternal thyroid hormone homeostasis in the rat. Toxicol Sci. 2002;68:361–371. doi: 10.1093/toxsci/68.2.361. [DOI] [PubMed] [Google Scholar]
- Moon RT, Morrill JB. The effects of Gymnodinium breve lysate on the larval development of the sea urchin Lytechinus variegates. J Environ Sci Health. 1976;A11(1011):673–683. [Google Scholar]
- Morgan DJ. Drug disposition in mother and foetus. Clin Exp Pharmacol Physiol. 1997;24:869–873. doi: 10.1111/j.1440-1681.1997.tb02707.x. [DOI] [PubMed] [Google Scholar]
- Naar J, Bourdelais A, Tomas C, Kubanek J, Whitney PL, Flewelling L, Steidinger K, Lancaster J, Baden DG. A competitive ELISA to detect brevetoxins from Karenia brevis (formerly Gymnodinium breve) in seawater, shellfish, and mammalian body fluid. Environ Health Perspect. 2002;110:179–185. doi: 10.1289/ehp.02110179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council. Guide for the Care and Use of Laboratory Animals. National Academies Press; Washington, DC: 1996. [Google Scholar]
- Needleman JL. Behavioral toxicology. Environ Health Perspect. 1995;103:77–79. doi: 10.1289/ehp.95103s677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera FP, Jedrychowski W, Rauh V, Whyatt RM. Molecular epidemiologic research on the effects of environmental pollutants on the fetus. Environ Health Perspect. 1999;107(Suppl 3):451–460. doi: 10.1289/ehp.99107s3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poli MA, Mende TJ, Baden DG. Brevetoxins, unique activators of voltage-sensitive sodium channels, bind to specific sites in rat brain synaptosomes. Mol Pharmacol. 1986;30(2):129–135. [PubMed] [Google Scholar]
- Poli MA, Templeton CB, Thompson WL, Hewetson JF. Distribution and elimination of brevetoxin-3 in rats. Toxicon. 1990a;28:903–910. doi: 10.1016/0041-0101(90)90020-8. [DOI] [PubMed] [Google Scholar]
- Poli MA, Templeton CB, Pace JG, Hines HB. Detection, metabolism, and pathophysiology of brevetoxins. In: Hall S, Strichartz G, editors. Marine Toxins Origin, Structure and Molecular Pharmacology. Vol. 418. American Chemical Society, ACS Symposium Series; Washington, DC: 1990b. pp. 176–191. [Google Scholar]
- Poli MA, Musser SM, Dickey RW, Eilers PP, Hall S. Neurotoxic shellfish poisoning and brevetoxin metabolites: A case study from Florida. Toxicon. 2000;38:981–993. doi: 10.1016/s0041-0101(99)00191-9. [DOI] [PubMed] [Google Scholar]
- Riley CM, Holt SA, Holt J, Burskey EJ, Arnold CR. Mortality of larval red drum (Sciaenops ocellatus) associated with a Ptychodiscus brevis red tide. Contrib Mar Sci. 1989;31:137–146. [Google Scholar]
- Rodier PM. Developing brain as a target of toxicity. Environ Health Perspect. 1995;103:63–66. doi: 10.1289/ehp.95103s673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman BL, Timms BG, Prins GS, Peterson RE. In utero and lactational exposure of the male rat to 2,3,7,8-tetrachlorodibenzo-p-dixoin impairs prostate development. 2. Effects on growth and cytodifferentiation. Toxicol Appl Pharmacol. 1998;150:254–270. doi: 10.1006/taap.1998.8395. [DOI] [PubMed] [Google Scholar]
- Sayer A, Hu Q, Bourdelais AJ, Baden DG, Gibson JE. The effect of brevenal on brevetoxin-induced DNA damage in human lymphocytes. Arch Toxicol. 2005;79:683–688. doi: 10.1007/s00204-005-0676-2. Epub 2005 Jun 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinjari T, Darnerud PO. Hydroxylated polychlorinated biphenyls: Placental transfer and effects on thyroxine in the foetal mouse. Xenobiotica. 1998;28:21–30. doi: 10.1080/004982598239722. [DOI] [PubMed] [Google Scholar]
- Tibbetts BM, Baden DG, Benson JM. Uptake, tissue distribution, and excretion of brevetoxin-3 administered to mice by intratracheal instillation. J Toxicol Environ Health Part A. 2006;69:1325–1335. doi: 10.1080/15287390500360091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unadkat JD, Dahlin A, Vijay S. Placental drug transporters. Curr Drug Metab. 2004;5:125–131. doi: 10.2174/1389200043489171. [DOI] [PubMed] [Google Scholar]
- Wang W, Hua Y, Wang G, Cole RB. Characterization of rat liver microsomal and hepatocytal metabolites of brevetoxins by liquid chromatography-electrospray tandem mass spectrometry. Anal Bioanal Chem. 2005;383:67–75. doi: 10.1007/s00216-005-3323-0. [DOI] [PubMed] [Google Scholar]
- You G. The role of organic ion transporters in drug disposition: an update. Curr Drug Metab. 2004;5(1):55–62. doi: 10.2174/1389200043489207. [DOI] [PubMed] [Google Scholar]