Abstract
Aquaporins (AQP) constitute the principal pathway for water movement across biological membranes. Consequently, their expression and function is important for cell volume regulation. Glioma cells quickly adjust their cell volume in response to osmotic challenges or spontaneously as they invade into the narrow and tortuous extracellular spaces of the brain. These cell volume changes are likely to engage water movements across the cell membrane through AQP. AQP expression in glioma cells is poorly understood. In this study, we examined the expression of AQP in several commonly used human glioma cell lines (D54, D65, STTG1, U87, U251) and in numerous acute patient biopsies by PCR, Western blot, and immunocytochemistry and compared them to nonmalignant astrocytes and normal brain. All glioma patient biopsies expressed AQP1, AQP4 and some expressed AQP5. However, when isolated and grown as cell lines they lose all AQP proteins except a few cell lines that maintain expression of AQP1 (D65, U251, GBM62). Reintroducing either AQP1 or AQP4 stably into glioma cell lines allowed us to show that each AQP is sufficient to restore water permeability. Yet, only the presence of AQP1, but not AQP4, enhanced cell growth and migration, typical properties of gliomas, while AQP4 enhanced cell adhesion suggesting differential biological roles for AQP1 and AQP4 in glioma cell biology.
Keywords: edema, glioma, volume regulation, migration
INTRODUCTION
The ability of cells to control their cell volume in the face of a changing osmotic environment is a fundamental property shared by many cells across species (Lang et al., 1998). Of particular importance is regulatory volume decrease (RVD) that allows cells to remove excess water from their cytoplasm in an attempt to maintain proper water balance. RVD has been shown to involve the release of organic and inorganic osmolytes into the extracellular space, which in turn leads to an obligatory efflux of water (Parkerson and Sontheimer, 2003, 2004). While the pathways for osmolyte release have been studied for several decades, the pathways for water movement across cell membranes have only recently been elucidated. These studies have demonstrated that water extrusion occurs through specialized water channels or AQP (Preston et al., 1992), although in some cells water permeates lipid membranes directly (Zeidel et al., 1992; Zhang et al., 1993) or passes the membrane through unrelated channels (Fischbarg et al., 1990; MacAulay et al., 2001).
Under physiological conditions the brain and spinal cord are thought to encounter few osmotic challenges since the composition of the cerebral spinal fluid fluctuates much less than the blood plasma from which it is separated by the blood-brain barrier. This is important as the tight packaging of neurons, glial cells, and blood vessels in brain would not permit significant changes in the volume occupied by individual cells. However, acute injury (Ke et al., 2001), stroke (Taniguchi et al., 2000), and inflammation (Alexander et al., 2003) each present with significant water uptake by brain cells resulting in significant brain swelling or edema that confound the neurological problem. Swelling also accompanies many primary and secondary brain tumors (Foncin and Le Beau, 1978) impeding the function of adjacent peritumoral brain. Peritumoral edema occurs as a consequence of a disrupted blood-brain barrier (Bothe et al., 1984; Hossman and Bloink, 1981). However, tumor growth appears to be unaffected by peritumoral edema and glioma cells actually appear to thrive in this edematous environment. We previously noted that glioma cells have a well-developed volume regulatory response that restores the cell volume even in the presence of significant osmotic challenges (Ernest et al., 2005). These studies suggest a major role for Cl- release through ion channels in this process. Pathways for water movement, however, had not been previously investigated and this study set out to fill this void. Water permeability and its role in glioma volume are also important in the context of glioma invasion. Unlike other cancers that spread hematogenously, gliomas invade the brain by active cell migration. We previously hypothesized that glioma cells may have an unusual ability to regulate their volume in order to fit through the tortuous extracellular brain spaces as they invade, which would require enhanced water permeability.
In this study, we examined aquaporin (AQP) expression by PCR and Western blot in a panel of frequently used glioma cell lines as well as in acute patient derived biopsies and cells derived from them. We show prominent expression of AQP1 and AQP4, and to a lesser extent AQP5. Surprisingly, only AQP1 expression was maintained in cultured glioma cells many of which lose AQP expression altogether. Reintroducing either AQP1 or AQP4 stable into glioma cell lines showed that each AQP is sufficient to restore water permeability such that cells expressing either AQP behave like perfect osmometers when challenged osmotically. On the other hand, the absence of AQP1 but not AQP4 impedes the ability of cells to invade. These studies therefore suggest differential roles for AQP1 and AQP4 in glioma cell biology.
MATERIALS AND METHODS
Cell Culture
U87-MG [astrocytoma, World Health Organization (WHO) grade III], STTG-1 (anaplastic astrocytoma, WHO grade III), and D65-MG (WHO grade IV) were all obtained from American Type Culture Collection (ATCC; Rockville, MD). D54-MG (WHO grade IV) and U251-MG (WHO grade IV) were a gift from Dr. D.D. Bigner (Duke University, Durham, NC). Glioblastoma multiforme 010701062 (GBM62; WHO grade IV) and glioblastoma multiforme 010601050 (GBM50; WHO grade IV) were glioblastoma cell lines cultured from patient biopsies (University of Alabama at Birmingham). Cells were grown in Dulbecco’s modified Eagle medium (DMEM/F12; Media Tech, University of Alabama at Birmingham Media Preparation Facility) and supplemented with 2 mM glutamine (Media Tech) and 7% heat-inactivated fetal bovine serum (FBS; Hyclone, Logan, UT) at 37°C and 90% O2/10% CO2 humidified environment. Human glioma tissue, astrocytoma tissue, and normal brain blocks were obtained from Cooperative Human Tissue Network (CHTN). Representative tissue samples were used in each of the following experiments.
RNA Isolation and PCR
Messenger RNA was extracted from the various cell lines following the RNAqueous protocol (Ambion, Austin, TX). Briefly, the cells were lysed, homogenized, and centrifuged. The supernatant was removed and an equal volume of 64% ethanol was added. The mixture was filtered via centrifugation and the filters were washed. The mRNA was eluted from the filter and DNA-free (Ambion) was used to remove contaminating DNA. RNA quality was evaluated by electrophoresis through 1.5% agarose gels.
The cDNA was synthesized and amplified using the OneStep RT-PCR kit (Qiagen, Valencia, CA), per manufacturer’s instructions, by the Eppendorf Mastercycler gradient (Brinkmann Instruments, Westbury, NY). Oligonucleotide primers (Invitrogen, Carlsbad, CA) were designed to amplify specific Aquaporins (AQPs) and used the sequences described previously (Wang et al., 2003) and listed in Table 1, and 2 ηg of mRNA was loaded per reaction. PCR conditions were as follows: denaturation at 94°C for 5 min followed by 35 cycles at 94°C for 30 s, 56°C for 30 s, 72°C for 2 min, with a final extension at 72°C for 10 min. Amplified products were electrophoresed through 1.5% agarose gels to determine size. Controls were used for all AQP. Kidney cDNA was used for AQP1, 2, 3, 4, 6, 7, 8, and lung cDNA was used for AQP5. Placental, small intestine, and liver cDNA were used for AQP0, AQP10, and AQP9, respectively. Actin was used as loading controls.
TABLE 1. Oligonucleotide Primer Sequences Used to Amplify Human Aquaporins via RT-PCR.
| Gene | Primer sequence (5′-3′) | Length |
|---|---|---|
| AQP0 | Forward ATT CTC ACT GGG AAC TTC ACT AAC Reverse AGG GCC TGG GAG TTC AGT TCA ACA |
203 |
| AQP1 | Forward GGC CAC GAC CCT CTT TGT CTT CAT Reverse TCC CAC AGC CAG TGT AGT CAA TAG |
514 |
| AQP2 | Forward AGC CGC TCT GCT CCA TGA GAT CAC Reverse GGC GGA AAC AGC ACG TAG TTG TAG |
375 |
| AQP3 | Forward TCA ATG GCT TCT TTG ACC AGT TCA Reverse CTT CAC ATG GGC CAG CTT CAC ATT |
389 |
| AQP4 | Forward CAT CGC CAA GTC TGT CTT CTA CAT Reverse GCT ATT GAG CCA GTG ACA TCA GTC |
237 |
| AQP5 | Forward CCT GTC CAT TGG CCT GTC TGT CAC Reverse GGC TCA TAC GTG CCT TTG ATG ATG |
225 |
| AQP6 | Forward GCA TCA TCA TTG GGA AGT TCA CAG Reverse GCG TAG GCT GAT TCA CAC ACT CTC |
251 |
| AQP7 | Forward AAA TGG TCT CCT GGT CCG TGA TAG Reverse ACA CCA AGG TAG CTC CCA AAT GTT |
178 |
| AQP8 | Forward TTG TGC CAT CTG ATC CTG ATG TCT Reverse GCA GCG TCG TCA GGA TGA TCT CTG |
520 |
| AQP9 | Forward CGG CAT TTG TAC AGT CAG AGA CTC Reverse AAT GCG TTC GCC AGA GAT AGA TAC |
632 |
| AQP10 | Forward TGG GGT TCC CTC TTC TAA ATA CTA Reverse CTC CCA GGT TCT GGC ACA TTA ACA |
450 |
Western Blot Analysis
A confluent dish of cells was lysed using RIPA buffer [(50 mM TrisCl, pH 7.5, 150 mM NaCl, 1% Nondet P-40 (NP-40), 0.5% sodium deoxycholate, 1% sodium dodecyl sulfate (SDS)] supplemented with protease inhibitor cocktail (Sigma). Cells were sonicated for 10 s and centrifuged at 14,000 rpm for 10 min, and the supernatant was transferred to a new tube. Protein quantification was performed using a DC protein assay kit (BioRad, Hercules, CA). An equal amount of 63 sample buffer containing 600 mM β-mercaptoethanol was added to the 20-30 μg/ml of cell lysate per lane. Samples were loaded into a 10% precast SDS-PAGE gel (BioRad). Protein separation was obtained using a constant 100 V for 80 min, and the gels were transferred at 200 mA for 2 h at room temperature onto polyvinylidine difluoride (PVDF) paper (Millipore, Bedford, MA). Membranes were blocked in blocking buffer (3% nonfat dried milk in TBS plus 0.1% Tween 20). All antibodies were obtained from Chemicon (Temecula, CA) and used following manufacturer’s instructions. Membranes were incubated in primary antibody for 1 h at room temperature and washed 3× for 10 min. The membranes were then incubated in horseradish peroxidase (HRP)-conjugated secondary antibodies (Sigma) at 1:1,000 for 1 h followed by another round of washing (3 × 10 min) and developed using Luminol (Santa Cruz, Santa Cruz, CA) on Hyperfilm (Amersham, Arlington Heights, IL).
Immunocytochemistry
Cells plated on coverslips were washed with phosphate-buffered saline (PBS) and fixed in 4% paraformaldehyde for 10 min. Cells were washed 2 × 10 min in PBS, blocked, and then permeabilized in PBS containing 0.3% Triton X-100 and 5% normal goat serum (NGS) for 30 min. Cells were incubated overnight at 4°C in primary AQP antibodies at 1:200. On the following day, glioma cells were washed in PBS (4 × 5 min), blocked again in PBS with 5% NGS, and then incubated with phalloidin (Molecular Probes, Eugene, OR) at 1:100 and fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit secondary antibody (Molecular Probes) diluted at 1:500 in the dark for 1 h. Cells were washed in PBS (2 × 5 min) and incubated with DAPI, a fluorescent nuclear label, (1:2,000, Sigma) for 5 min. Cells were washed two more times with PBS and mounted on slides with GelMount (Biomedia, Foster City, CA). Images were acquired using an inverted Olympus IX-81 spinning disk confocal microscope (Olympus, Center Valley, PA).
Cryostat sections of human glioma tissue, astrocytoma tissue, and normal brain were cut at 8 μm and fixed with 4% paraformaldehyde. Cells were washed 2× in PBS and permeabilized in permeabilization solution (3% goat serum, 0.03% Triton X-100 in PBS). Sections were washed 2× for 5 min in PBS and blocked for 1 h in blocking solution (5% goat serum in PBS). Sections were then incubated overnight at 4°C in primary AQP (1:100; Chemicon) and GFAP (1:1,000; Sigma) antibodies in incubation solution (1% goat serum, 0.25% Triton X-100 in PBS). After overnight incubation, sections were washed 3× for 10 min in PBS and incubated for 2 h at RT in the dark in incubation solution. Sections were then washed 2× for 10 min in PBS, DAPI (1:2,000; Sigma) was applied for 5 min, and followed by a final wash in PBS for 10 min. Sections were mounted on slides using GelMount (Biomedia).
Transfections
The AQP1-GFP plasmid was a gift from Dr. Nicholas LaRusso (Mayo Clinic, Minneapolis, MN), and AQP4-DsRed was a gift from Dr. Ken-ichi Nakahama (Tokyo Medical and Dental University, Tokyo, Japan). AQP1 and AQP4 constructs were created as discussed in published work, i.e., (Tietz et al., 2006) for AQP1 and (Nakahama et al., 2002) for AQP4. D54 tumor cells were transfected with AQP1-GFP or eGFP-N1 (Clontech, Mountain View, CA) and AQP4-DsRed or DsRed-N1 (Clontech) using Nucleofector Kit T (Amaxa, Gaithersburg, MD). On the day of transfection, cells were harvested and 2 × 106 cells were mixed with 2 μg of plasmid in 100 μL of the nucleofector solution and then electroporated with the Amaxa Nucleofector (Amaxa). Electroporation of cells was completed with Amaxa program T-27. This suspension was transferred to an eppendorf tube and diluted appropriately for plating. After 48 h, cells were treated with 1 mg/mL geneticin (Sigma) and each week the concentration was reduced until reaching 250 μg/mL. Individual cells were sorted into 96-well plates by FACS and colonies were selected from single clones. Stable cells were maintained using 250 μg/mL of geneticin.
AQP1-eGFP was constructed by ligating annealed oligonucleotides 5′-GATCCCCGGGTGGAGATGAAGCCCAAATTTCAAGAGAATTTGGGCTTCATCTCCACCCTTTTTG-3′ and 3′-GGGCCCACCTCTACTTTCGGGTTTAAAGTTCTCTTAAACCCGAAGTAGAGGTGGGAAAAACAGCT-5′ at BglII and SalI restriction sites of the pZOFF-EGFP plasmid. AQP1-shRNA was transfected using FuGene (Roche Diagnostics, Indianapolis, IN). FuGene was incubated for 5 min in 100 μL serum-free media. DNA was added, incubated for 35 min, dispersed onto 1 × 106 cells, and cells were allowed to recover for 48 h.
Volume Regulation
Cell volume measurements were performed using a Coulter Counter Multisizer 3 (Beckman-Coulter, Miami, FL) as described previously (Parkerson and Sontheimer, 2003). Cells were washed in PBS and lifted from the dish using 0.05% trypsin and 0.53 mM EDTA. Trypsin was inactivated with the addition of an equal volume of serum-containing media and cells were briefly centrifuged to pellet. Cells were resuspended in bath solution [125 mM NaCl, 5.0 mM KCl, 1.2 mM MgSO4, 1.6 mM Na2HPO4, 0.4 mM NaH2PO4, 10.5 mM glucose, 32.5 mM HEPES (acid), 1.0 mM CaCl2, pH 7.4, 300 ± 10 mos-mol]. Osmolarity for solutions was measured by a freeze point osmometer (Fiske Micro-Osmometer 210; Fiske-Associates, Norwood, MA). Cells were equilibrated for ∼5 min before first reading and readings were taken continuously for 3 min. All experiments contained 200 μM 5-nitro-2-(3-phenylpropylamino)-benzoic acid (NPPB, Sigma) and 250 μM CdCl2. Bath solution was made hyposmotic with the addition of water. Mercuric chloride (HgCl2, Sigma) was made at 300 μM in bath solution and cells were preincubated for 5 min. Data were collected by Multisizer 3 software, and 5,000 pulse listings were exported to EXCEL as the average of 40-50 cells for each 20 ms time point. Data were collected as mean diameter and were converted to mean cell volume. Mean cell volumes were normalized to baseline values. Data were plotted in Origin 7.0 (MicroCal, Northhampton, MA) ± se with (n) experiments performed. Each time point graphed is an average of the mean cell volume for 40-50 cells per 20 ms. Each graph for a 3 min experiment contained 5,000 data points.
Cell Migration
Migration was assessed using a modified Boyden Chamber. Eight-micrometer FluorBlok filters (Fisher Scientific, Pittsburgh, PA) were coated with 2.5 mg/mL vitronectin (Sigma) overnight at room temperature. Filters were washed twice with PBS and blocked for 1 h at 37°C with 1% fatty acid free BSA in PBS. Cells were trypsinized using 0.5 mM EGTA and centrifuged. Cells were washed twice with PBS and brought up in migration assay buffer (serum-free DMEM/F12 with 0.1% fatty acid free BSA). Cells were counted using a hemocytometer and 40,000 cells were plated per filter and allowed to migrate for 5 h. Cells were fixed in 4% paraformaldehyde for 10 min followed by two washes in PBS. DAPI was applied at 1:2,000 for 5 min followed by an additional two washes in PBS. For shRNA experiments, the top and the bottom of each filter were counted and a comparison was made between migrated and non-migrated cells. Images of five random fields were taken using Zeiss Axiovert 200M (München, Germany). All experiments were performed in triplicate.
Cell Adhesion Assay
Coverslips were coated overnight at 4°C with various matrices: 10 μg/mL collagen I (Sigma), 10 μg/mL fibronectin III (Sigma), 20 μg/mL laminin (Sigma), 20 μg/mL vitronectin (Sigma), or 1% BSA (Sigma). Two hundred thousand cells were seeded per well and allowed to adhere for 1 h at 37°C. The nonadherent cells were washed away gently using PBS and cells were fixed using 4% paraformaldehyde. Images of five random fields were taken using Zeiss Axiovert 200M (München, Germany) at 10× magnification.
RESULTS
RT-PCR Demonstrates Distinct AQP Transcripts in Glioma Cells
The principal objective of this study was to examine the contribution of AQP to trans-membrane water transport and hence cell volume changes in glioma cells. As a first step towards this goal we used RT-PCR to search for the complement of AQP genes expressed. We examined a rather broad range of cells and tissues including five commonly used glioma cell lines (D54, D65, STTG1, U87, U251), glioma cultures from two patient samples (GBM50 and GBM62), and a selection of acute patient biopsy tissues (Normal Brain, Astrocytoma and Glioblastoma, Fig. 1). Primers for each aquaporin are listed in Table 1. The most abundant transcripts were those for AQP1, 3, 5 and 6, which were found in most cell types. AQP4 mRNA similar appeared in most cell lines, but highest levels were detected in normal brain and glioma patient biopsies (Fig. 1). As for other AQPs, expression varied from cell type to cell type. AQP7 mRNA was not detected in any glioma cell type (Fig. 1) and only the D54-MG cells showed mRNA expression of AQP2 albeit at a low level. We did not see significant variations in the mRNA expression between the anaplastic astrocytoma and glioblastoma patient biopsies with the exception of AQP1 (Fig. 1). Expression levels of AQP1 in GBM tissue was significantly higher than any other tissue sample we examined confirming the findings from previous work (Oshio et al., 2005). All AQP transcripts found in the glioblastoma biopsies were also found in at least one of the glioma cell lines; of these U87 and U251 had an expression pattern more similar to patient biopsies.
Fig. 1.
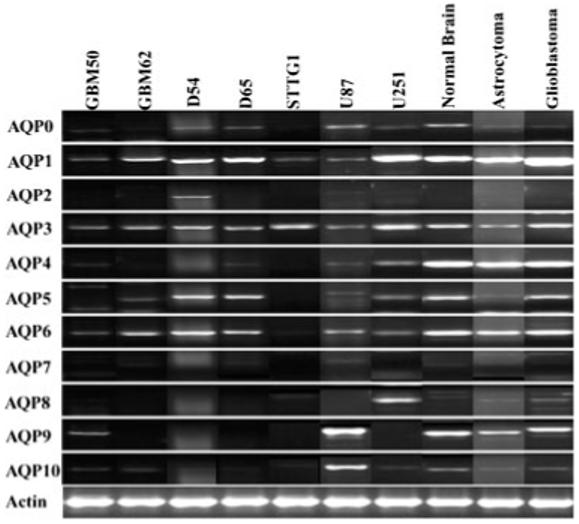
Aquaporin mRNA expression in astrocytes, several commonly used human glioma cell lines and acute patient biopsies (n = 6). RT-PCR was used to screen for all AQPs present in each cell type. All panels show a 1.5% agarose gel stained with ethidium bromide following amplification. Primers for each AQP are described in Table 1. AQP1, AQP5, and AQP6 were ubiquitously expressed in all cell types, but there was variable distribution of all other AQPs. There was no expression of AQP7 in any of the different cell types, but only the D54-WT showed expression of AQP2. Positive controls for each AQP were used and are described in Materials and Methods.
Differences in Aquaporin Protein Expression Between Glioma Cell Lines and Patient Biopsies
We next sought to examine that of the aquaporin transcripts actually yielded aquaporin protein by performing Western blot analyses (Fig. 2). Each human glioma cell line was probed with the AQP antibodies to the candidate genes determined by RT-PCR. Surprisingly, AQP1 was absent in GBM50, D54, STTG1, and U87 cells (Fig. 2A) but expressed in GBM62 and D65 cells where it occurred as a 28 kD band and a second presumably glycosylated protein band around 38 kD. In U251 cells, AQP1 antibodies only identified the 28 kD band. All cell lines lacked detectable levels of AQP4 and AQP5. For individual patient biopsies (Fig. 2B) expression pattern followed much more closely than predicted by RT-PCR with human anaplastic astocytomas and glioblastomas each expressing AQP1 and AQP4 at high levels. A majority of glioblastoma tissues samples examined expressed only the 32 kD band of AQP4 indicating a high expression of M23 isoform. Normal brain showed similar prominent expression of AQP1 and AQP4. This was in stark contrast to the glioma cell lines, which did not express AQP4 and showed variable AQP1 expression. While low levels of AQP5 was observed in normal brain tissue, two of the three primary glioblastomas revealed high AQP5 expression and one of three astrocytomas showed high expression levels.
Fig. 2.
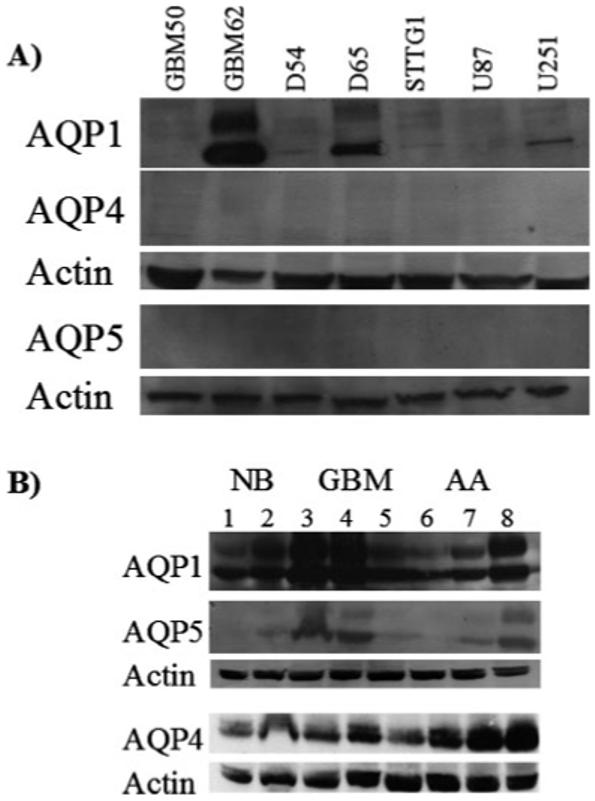
Western blot analysis of aquaporin recognized by RT-PCR. Cell lysates were separated using 10% SDS-PAGE gels and transferred onto PVDF paper and probed with antibodies to AQPs previously detected from RT-PCR experiments. (A) Only AQP1 was expressed in the human glioma cell lines, GBM62, D65, and U251. (B) Several tissue biopsies and tumor grades were examined showing expression of AQP1, AQP4, and AQP5 (NB, normal brain n = 5, lanes 1, 2; GBM, glioblastoma multiforme n = 6, lanes 3-5; AA, anaplastic astrocytoma n = 6, lanes 6-8).
Aquaporins Show Distinct Subcellular Localizations
Aquaporin expression on the plasma membrane is necessary for a participating role in water homeostatic mechanisms. To determine aquaporin localization, cellular and subcellular, we immunostained glioma cell cultures and patient tissue sections with antibodies to AQP1 and AQP4. Representative fluorescent images were taken at 60× using a spinning disk confocal microscope and z-stack images are illustrated in Figs. 3 and 4. Moderate AQP1 staining was found throughout GBM62 cells (Fig. 3A) with prominent AQP1 expression on the surface membrane, especially at the ruffled processes (Fig. 3B). Conversely, expression of AQP1 was not observed in D54 cells (Fig. 3A), and neither GBM62 nor D54 cultures showed expression of AQP4 (not shown), which is consistent with Western blot (Fig. 2A). In contrast and in accordance with Western blot data (Fig. 2B), acute patient glioblastoma tissue showed expression of AQP1, AQP4, and AQP5, which was localized throughout the tissue sample with regions of high expression (Fig. 4). AQP5 showed expression along fibrous tracts, possibly along the cytoskeleton, which was not found in either AQP1 or AQP4 stained tissue sections. The distribution of AQP1 and AQP4 was similar with expression throughout the tissue. However, all AQPs were found to be heterogeneously expressed throughout the tissue section consistent with previous studies (Endo et al., 1999; Saadoun et al., 2002a,b).
Fig. 3.
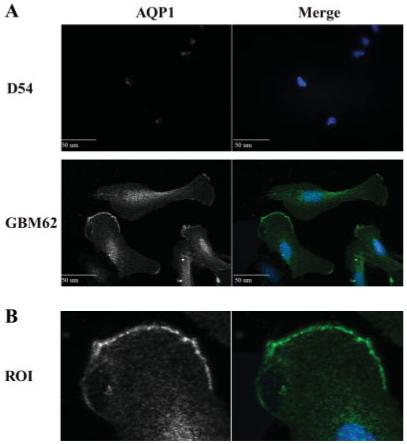
Localization of AQP1 using immunofluorescence. (A) Glioma cell lines were stained with anti-AQP and DAP1 as described in Materials and Methods. D54 cells do not show any expression of AQP1. GBM62 also shows expression of AQP1 throughout the cell body with distinct localization at the leading process (B).
Fig. 4.
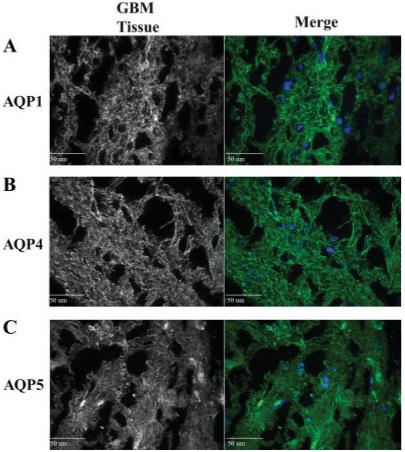
Localization of AQP1, AQP4 and AQP5 using immunofluorescence as described in Figure 3. (A) Glioma tissue sections show expression of AQP1 throughout the tissue with regions of high expression levels. (B) AQP4 shows little or no expression in any glioma cell line. As with AQP1, AQP4 is expressed throughout the glioma tissue sections. (C) AQP5 expression pattern is similar to AQP4.
AQP1 & AQP4 Enhance Water Permeability in Glioma Cells
Taken the above data together suggests prominent expression of AQP1 and AQP4 in glioma biopsies but loss of AQP proteins in long-term glioma cultures and cell lines. While unexpected, it gives us an opportunity to examine, through over-expression of recombinant AQPs, their contribution to water transport and their possible role in cell functions engaging water movement. In agreement with previous studies (Endo et al., 1999; Markert et al., 2001; Saadoun et al., 2002a,b), we have determined that AQP1 and AQP4 are both upregulated in glioma tissue. To this end we obtained clones for AQP1 & AQP4, the two proteins consistently expressed in patient derived biopsies and expressed them individually in D54 glioma cells that lack endogenous AQPs. Following transfection with a N1-plasmid containing AQP1-GFP or AQP4-DsRed, we generated stable cell lines (Fig. 5) that selectively express AQP1 and AQP4, respectively, henceforth termed D54-AQP1 and D54-AQP4. These showed prominent expression of the respective protein by Western blot and immuno staining (Figs. 5A,B) which was absent in D54-WT, wildtype, cells. We next examined water transport directly by measuring cell swelling in response to a hypoosmotic challenge with a Coulter-Counter Cell Sizer. Cell size was measured continuously, with 20 ms time resolution over a 3 min period during which a 60 s baseline volume was followed by the addition of a 50% hypoosmotic challenge and recorded for an additional 2 min (see methods for detail). Representative examples are shown in Fig. 5 in which the average cell volume ± S.E.M. for 1,000 cells each was plotted every 20 ms. It was difficult to quantitatively assess the water permeability in D54-MG cells as the maximal swelling response is counteracted by RVD. To prevent RVD in D54-MG cells, we used Cl- channel blockers, 200 μM NPPB and 250 μM Cd2+ at 15°C since in glioma cells RVD engages a combination of Cl- channels and KCC transport (Ernest et al., 2005). This resulted in cells behaving like perfect osmometers and hence allowing us to isolate and compare more quantitatively the water transport in cells expressing either AQP1 or AQP4, respectively. Figs. 5C,D show volume changes caused by water movement in D54 glioma cells expressing either AQP1 or AQP4. From these data we derived the time constant (τ) of the exponential of the rise in volume and plotted the reciprocal, which would be proportional to the water permeability (Fig. 5E). This data suggests that D54-AQP1 cells have enhanced water permeability over wildtype D54 by 98% similar to D54-AQP4 expressing cells (96%, Fig. 5E). D54-AQP1 cells in which AQP function was inhibited by 300 μM HgCl2 (Fig. 5C), (Jung et al., 1994), showed a reduced rate of cell swelling comparable to that of D54-WT that lack AQP1 (Fig. 5E). The rate of swelling seen in D54-AQP1 cells was similar to patient derived GBM62 cells containing endogenous AQP1 and these cells also show mercury sensitivity (data not shown). As expected, HgCl2 did not inhibit swelling in AQP4-expressing cells (Fig. 5D,E), which is expected since AQP4 is insensitive to mercury. Both D54-AQP1 and D54-AQP4 cells did not show much temperature dependence of water transport unless cooled to 4°C (data not shown) which is in line with previous studies (Folkesson et al., 1994; Roberts et al., 1994; Zhang et al., 1993). Note that differences in the degree of swelling were only observed in the initial minutes following the challenge as ultimately, as expected, both cell types reached similar cell volumes.
Fig. 5.
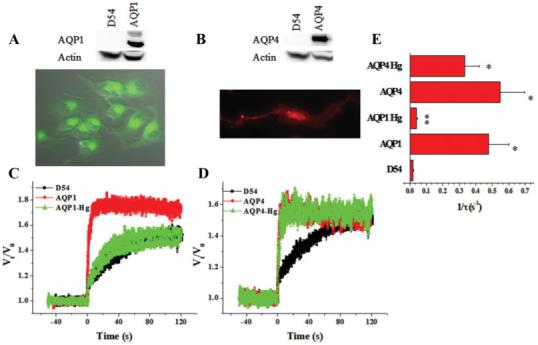
Function and expression of AQP1 and AQP4 in D54 glioma cells. (A,B) Western blot and 20× image of AQP1 and AQP4 expressing D54 cells. (C,D) Mean cell volume was measured for 3 min in D54-WT and AQP1-D54 cells (n = 5-7) and AQP4-D54 cells (n = 4-7) following a 50% hyposmotic challenge in the presence of 200 μM NPPB and 250 μM CdCl2. HgCl2 was used at 300 μM. (E) Reciprocal exponential time constant (τ-1), which is proportional to osmotic water permeability. (Significance was assessed using an ANOVA *P < 0.05 as compared to control. **P < 0.05 as compared to AQP1). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
D54-AQP1 Show Enhanced Migration
In light of the hypothesized role of water channels in volume regulation associated with cell migration, we next set out to examine the relative role of AQP1 & AQP4 in migration. Transwell assays are a convenient assay system to mimic the spatial constraints of the extracellular space. We plated 40,000 cells onto each 8 μm filter and cells were allowed to migrate for 5 h at which time the cells were fixed and images taken for analysis. We found that compared to wildtype cells ∼twice as many AQP1 overexpressing cells had migrated across the filters (Fig. 6A). Indeed, the absolute number of cells that successfully migrated was comparable to the patient derived GBM62 cells that maintained endogenous AQP1 (Av. 162± versus 181± migrated cells/5 h). If this were related to AQP1 function, one would expect that a decrease in AQP1 expression in the latter cell lines should cause a decrease in cell migration. Knock down of AQP1 expression using specific shRNA constructs (see methods) indeed caused a 55% reduction in protein expression and a 70% reduction in cell migration (Fig. 6C,D). This data suggests that AQP1 function enhances migration of glioma cells. When we more closely examined AQP1 localization in actively migrating cells, we found the protein to localize to the leading edge of the cells as shown for two representative cells at different stages of penetrating the Transwell pores (Fig. 6B). We subjected AQP4-expressing D54-AQP4 cells to the same migration assay and surprisingly found a reduction in migration of ∼40% (Fig. 6E). However, as with D54-AQP1 cells AQP4 still localized to the leading edge of migrating cells (Fig. 6F). This supports the findings from a recent study where migrating grade IV glioblastoma cells showed reduced AQP4 expression (Warth et al., 2007).
Fig. 6.
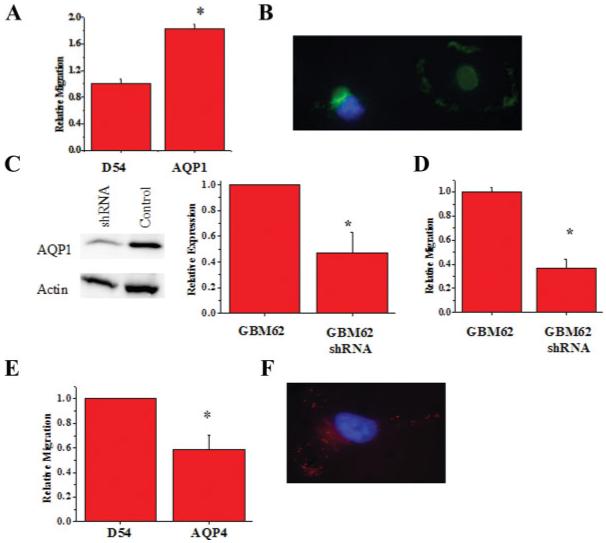
The role of AQPs in invasion. (A) Transwell migration assay comparing GFP-D54 cells and AQP1-D54 cells. Forty-thousand cells were allowed to migrate for 5 h through 8 μm FluorBlok filters. (B) Western blot showing knockdown of AQP1 using shRNA and (C)40× image of migrating AQP1-expressing D54 cell. (D) shRNA knockdown of AQP1 in D65 and GBM62 glioma cell lines (n = 3). (E) Comparison of DsRed-D54 and AQP4-D54 cells following migration. (F)40× image of AQP4-expressing D54 cell. (Significance was determined using a Student’s t-test; P < 0.05). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
D54-AQP4 Show Enhanced Cell Adhesion
Migration requires cells to dynamically alter their adhesiveness to their growth substrate (Uhm et al., 1999) hence any changes in migration may be secondary to altered cell adhesiveness. To question the possibility that AQP1 expression may enhance cell migration by reducing cell adhesion, we examined this question directly by coating coverslips with several common extracellular matrices (collagen, fibronectin, laminin, and vitronectin), allowing cells to adhere for 1 h, and gently washing away any non-adherent cells. Adherent cells were quantified and compared with control D54 cells. As illustrated in Fig. 7, there was no significant difference in adhesion when comparing the D54-AQP1 to control D54-WT on the various substrates. However, there was an overall increase in cell adhesion for D54-AQP4 cells for all substrates examined, which may at least in part explain the reduced difference of the cells to migrate (see above).
Fig. 7.
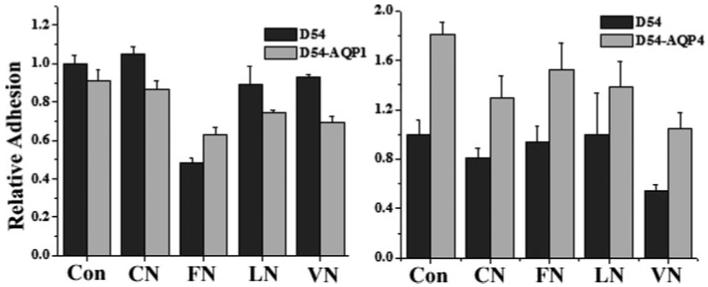
The role of AQPs in cell adhesion. To measure adherence, 200,000 cells were plated onto various matrices [1% BSA (Con), 10 μg/mL collagen I (CN), 10 μg/mL fibronectin III (FN), 20 μg/mL laminin (LN), 20 μg/mL vitronectin (VN)] for 1 h. Cells were gently washed away and fixed. Five random images were taken and counted. (Significance was determined using ANOVA; P < 0.05).
DISCUSSION
In this investigation, we were able to demonstrate the expression of AQP1 and AQP4 in all glioma patient biopsies examined by PCR, Western blot, and immunostaining. This finding largely supports previous studies showing expression of AQP1 and AQP4 (Endo et al., 1999; Markert et al., 2001; Saadoun et al., 2002a,b). Surprisingly, we observed the loss of one or all of the AQPs in cell lines established from human gliomas including those cell lines frequently investigated in glioma research. Importantly, AQP1 expression is retained in many of the glioma cultures, but AQP4 was lost in all glioma lines, even in cell lines derived from acute glioblastoma tissue. This loss is likely a result of culture condition. For example, it has been shown by several groups that some AQP are regulated via osmotic response elements and hypertonicity (Herrlich et al., 2004; Hoffert et al., 2000; Jenq et al., 1999; Umenishi and Schrier 2002, 2003). Cells not stimulated by constant changes in osmolarity may selectively downregulate AQP. AQP1 is upregulated by hypertonic challenge in kidney cells lacking endogenous expression of AQP1 (Umenishi and Schrier, 2003). In the absence of osmotic challenges, AQP1 expression may become superfluous in cultured cells and is downregulated. While AQP4 does not contain an osmotic response element (ORE), it may be affected by other artificial culture conditions.
The lack of AQP expression in many cell lines offered us an opportunity to study their relative contribution to water permeability in gliomas. By reconstituting the expression of AQPs through recombinant expression we were able to examine the role of each individual AQP. This technique was important because functional studies of AQPs in primary cells have been difficult since specific drugs that modulate their function are not available. The use of high-speed, real-time volumetric measurements confirmed that expression of either AQP1 or AQP4 resulted in a dramatic increase in water permeability. This allowed us to question the importance of AQP mediated water transport in important cell biological functions, specifically cell migration, a pronounced feature of glioblastomas.
We previously hypothesized that the invasive migration of glioma cells requires cells to undergo coordinated cell volume changes, most notably shrinkage as cells invade into narrow extracellular spaces in brain (Sontheimer, 2004). Specifically, we showed that secretion of Cl- and K+ is required for cell invasion (McFerrin and Sontheimer, 2006; Ransom et al., 2001). Furthermore, it was demonstrated that Ca2+-activated K+ channels, i.e. BK (Weaver et al., 2004) colocalize to lipid raft domains on invadipodia (McFerrin and Sontheimer, 2006). Our immunohistochemical studies also localize AQP1 and AQP4 to the leading edge of migrating tumor cells. We suggest that while Cl- and K+ channels provide the pathways for KCl secretion, AQP1 enhances the release of obligated water, which in turn causes the invading process of the cell to shrink. Consistent with this hypothesis, overexpression of AQP1 doubled the rate of cell migration through a transwell barrier that mimics the spatial constraints of brain. In further support of this argument, previous observations show that AQP1 expression also enhances the migration of melanoma cells (Hu and Verkman, 2006). Although AQP4 is also localized to invadipodia and similarly increases water permeability in glioma cells, we were surprised to find that it actually reduced the cell’s ability to migrate. This discrepancy may be due to our use of the M23 splice variant of AQP4, which has been suggested to increase cell adhesiveness (Hiroaki et al., 2006). As our GBM tissue endogenously expressed high levels of the M23 splice variant as compared with the M1, it is possible that AQP4 itself is not utilized for motility. Another possible hypothesis is that AQP4 colocalizes with a different subset of ion channels than AQP1. We know that D54 gliomas express several K+ channels (Bordey et al., 2000; Olsen and Sontheimer, 2004; Ransom and Sontheimer, 2001) and Cl- channels (Olsen et al., 2003). AQP1 expression may act in concert with one set of ion channels to enhance migration while AQP4 may interact with a different subset important for maintaining proper cell size. Of note, AQP1 and AQP4 may have similar differential roles in non-malignant glia. For example, during development, nonmalignant astrocytes lose expression of AQP1 and express only AQP4. The development of this expression pattern correlates with differentiated astrocytes also becoming stationary cells. Similarly, AQP play equally varied roles in many cell types (Echevarria et al., 1994; Nielsen et al., 1993).
Another hypothesis that may explain the differing functionality of AQP4 and AQP1 may be a separation of domains. Each AQP may localize within certain isolated regions, along with their respective ion channels, which are necessary to promote a particular cellular function, i.e., migration or adhesion. Specifically, AQP1 has a heterogeneous expression in primary glioblastoma tissue (Endo et al., 1999) indicating regions for rapid water movement. These regions may be indicative of areas of high migration. If AQP1 and AQP4 are indeed localized to different domains, then it is possible that they may also interact with different growth factor receptors. We know that the EGFR is upregulated in glioblastomas (Di Carlo et al., 1992), and EGF has been shown to increase VEGF secretion (Goldman et al., 1993; Tsai et al., 1995) and regulate proliferation (Engebraaten et al., 1993). It is possible that gliomas containing high expression of AQP1 may have higher levels of certain growth factors and their respective receptors leading to a switch between docile tumor growth (AQP4-expressing), and a highly migratory and rapidly growing (AQP1-expressing) tumor.
The differential effects of AQP1 and AQP4 expression on glioma cell biology are surprising. Clearly, further studies are needed to examine a mechanistic link between AQP function and the underlying biology. While we hypothesize that water flux is the primary function in migrating or proliferating cells, AQPs may have alternative, less obvious functions in this regard.
ACKNOWLEDGMENTS
We would like to thank Dr. Yancey Gillespie (University of Alabama-Birmingham) for providing us with cells from patient biopsies, Dr. Nicholas LaRusso (Mayo Clinic) for providing the AQP1-eGFP construct, and Dr. Ken-ichi Nakahama for providing AQP4 construct.
Grant sponsor: NIH; Grant number: NIH-RO1-NS36692.
REFERENCES
- Alexander JJ, Bao L, Jacob A, Kraus DM, Holers VM, Quigg RJ. Administration of the soluble complement inhibitor, Crry-Ig, reduces inflammation and aquaporin 4 expression in lupus cerebritis. Biochim Biophys Acta. 2003;1639:169–176. doi: 10.1016/j.bbadis.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Bordey A, Sontheimer H, Trouslard J. Muscarinic activation of bk channels induces membrane oscillations in glioma cells and leads to inhibition of cell migration [in process citation] J Membr Biol. 2000;176:31–40. doi: 10.1007/s00232001073. [DOI] [PubMed] [Google Scholar]
- Bothe HW, Bodsch W, Hossmann KA. Relationship between specific gravity, water content, and serum protein extravasation in various types of vasogenic brain edema. Acta Neuropathol (Berl) 1984;64:37–42. doi: 10.1007/BF00695604. [DOI] [PubMed] [Google Scholar]
- Di Carlo A, Mariano A, Macchia PE, Moroni MC, Beguinot L, Macchia V. Epidermal growth factor receptor in human brain tumors. J Endocrinol Invest. 1992;15:31–37. doi: 10.1007/BF03348650. [DOI] [PubMed] [Google Scholar]
- Echevarria M, Windhager EE, Tate SS, Frindt G. Cloning and expression of AQP3, a water channel from the medullary collecting duct of rat kidney. Proc Natl Acad Sci USA. 1994;91:10997–11001. doi: 10.1073/pnas.91.23.10997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo M, Jain RK, Witwer B, Brown D. Water channel (aquaporin 1) expression and distribution in mammary carcinomas and glioblastomas. Microvasc Res. 1999;58:89–98. doi: 10.1006/mvre.1999.2158. [DOI] [PubMed] [Google Scholar]
- Engebraaten O, Bjerkvig R, Pedersen PH, Laerum OD. Effects of EGF, bFGF, NGF and PDGF(bb) on cell proliferative, migratory and invasive capacities of human brain-tumour biopsies in vitro. Int J Cancer. 1993;53:209–214. doi: 10.1002/ijc.2910530206. [DOI] [PubMed] [Google Scholar]
- Ernest NJ, Weaver AK, Van Duyn LB, Sontheimer HW. Relative contribution of chloride channels and transporters to regulatory volume decrease in human glioma cells. Am J Physiol Cell Physiol. 2005;288:C1451–C1460. doi: 10.1152/ajpcell.00503.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischbarg J, Kuang KY, Vera JC, Arant S, Silverstein SC, Loike J, Rosen OM. Glucose transporters serve as water channels. Proc Natl Acad Sci USA. 1990;87:3244–3247. doi: 10.1073/pnas.87.8.3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkesson HG, Matthay MA, Hasegawa H, Kheradmand F, Verkman AS. Transcellular water transport in lung alveolar epithelium through mercury-sensitive water channels. Proc Natl Acad Sci USA. 1994;91:4970–4974. doi: 10.1073/pnas.91.11.4970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foncin JF, Le Beau J. The brain surrounding malignant gliomas: An ultrastructural study. Acta Neurochir (Wien) 1978;42:33–43. doi: 10.1007/BF01406629. [DOI] [PubMed] [Google Scholar]
- Goldman CK, Kim J, Wong WL, King V, Brock T, Gillespie GY. Epidermal growth factor stimulates vascular endothelial growth factor production by human malignant glioma cells: A model of glioblastoma multiforme pathophysiology. Mol Biol Cell. 1993;4:121–133. doi: 10.1091/mbc.4.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrlich A, Leitch V, King LS. Role of proneuregulin 1 cleavage and human epidermal growth factor receptor activation in hypertonic aquaporin induction. Proc Natl Acad Sci USA. 2004;101:15799–15804. doi: 10.1073/pnas.0406853101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiroaki Y, Tani K, Kamegawa A, Gyobu N, Nishikawa K, Suzuki H, Walz T, Sasaki S, Mitsuoka K, Kimura K, Mizoguchi A, Fujiyoshi Y. Implications of the aquaporin-4 structure on array formation and cell adhesion. J Mol Biol. 2006;355:628–639. doi: 10.1016/j.jmb.2005.10.081. [DOI] [PubMed] [Google Scholar]
- Hoffert JD, Leitch V, Agre P, King LS. Hypertonic induction of aquaporin-5 expression through an ERK-dependent pathway. J Biol Chem. 2000;275:9070–9077. doi: 10.1074/jbc.275.12.9070. [DOI] [PubMed] [Google Scholar]
- Hossman KA, Bloink M. Blood flow and regulation of blood flow in experimental peritumoral edema. Stroke. 1981;12:211–217. doi: 10.1161/01.str.12.2.211. [DOI] [PubMed] [Google Scholar]
- Hu J, Verkman AS. Increased migration and metastatic potential of tumor cells expressing aquaporin water channels. FASEB J. 2006;20:1892–1894. doi: 10.1096/fj.06-5930fje. [DOI] [PubMed] [Google Scholar]
- Jenq W, Cooper DR, Bittle P, Ramirez G. Aquaporin-1 expression in proximal tubule epithelial cells of human kidney is regulated by hyperosmolarity and contrast agents. Biochem Biophys Res Commun. 1999;256:240–248. doi: 10.1006/bbrc.1999.0306. [DOI] [PubMed] [Google Scholar]
- Jung JS, Preston GM, Smith BL, Guggino WB, Agre P. Molecular structure of the water channel through aquaporin CHIP. The hour-glass model. J Biol Chem. 1994;269:14648–14654. [PubMed] [Google Scholar]
- Ke C, Poon WS, Ng HK, Pang JC, Chan Y. Heterogeneous responses of aquaporin-4 in oedema formation in a replicated severe traumatic brain injury model in rats. Neurosci Lett. 2001;301:21–24. doi: 10.1016/s0304-3940(01)01589-0. [DOI] [PubMed] [Google Scholar]
- Lang F, Busch GL, Ritter M, Volkl H, Waldegger S, Gulbins E, Haussinger D. Functional significance of cell volume regulatory mechanisms. Physiol Rev. 1998;78:247–306. doi: 10.1152/physrev.1998.78.1.247. [DOI] [PubMed] [Google Scholar]
- MacAulay N, Gether U, Klaerke DA, Zeuthen T. Water transport by the human Na+-coupled glutamate cotransporter expressed in Xenopus oocytes. J Physiol. 2001;530:3–78. doi: 10.1111/j.1469-7793.2001.0367k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markert JM, Fuller CM, Gillespie GY, Bubien JK, McLean LA, Hong RL, Lee K, Gullans SR, Mapstone TB, Benos DJ. Differential gene expression profiling in human brain tumors. Physiol Genomics. 2001;5:21–33. doi: 10.1152/physiolgenomics.2001.5.1.21. [DOI] [PubMed] [Google Scholar]
- McFerrin MB, Sontheimer H. A role for ion channels in glioma cell invasion. Neuron Glia Biol. 2006;2:39–49. doi: 10.1017/S17440925X06000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahama K, Fujioka A, Nagano M, Satoh S, Furukawa K, Sasaki H, Shigeyoshi Y. A role of the C-terminus of aquaporin 4 in its membrane expression in cultured astrocytes. Genes Cells. 2002;7:731–741. doi: 10.1046/j.1365-2443.2002.00553.x. [DOI] [PubMed] [Google Scholar]
- Nielsen S, DiGiovanni SR, Christensen EI, Knepper MA, Harris HW. Cellular and subcellular immunolocalization of vasopressin-regulated water channel in rat kidney. Proc Natl Acad Sci USA. 1993;90:11663–11667. doi: 10.1073/pnas.90.24.11663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen ML, Schade S, Lyons SA, Amarillo MD, Sontheimer H. Expresssion of voltage-gated chloride channels in human glioma cells. J Neurosci. 2003;23:5572–5582. doi: 10.1523/JNEUROSCI.23-13-05572.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen ML, Sontheimer H. Mislocalization of Kir channels in malignant glia. Glia. 2004;46:63–73. doi: 10.1002/glia.10346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshio K, Binder DK, Liang Y, Bollen A, Feuerstein B, Berger MS, Manley GT. Expression of the aquaporin-1 water channel in human glial tumors. Neurosurgery. 2005;56:375–381. doi: 10.1227/01.neu.0000148904.57841.6b. [DOI] [PubMed] [Google Scholar]
- Parkerson KA, Sontheimer H. Contribution of chloride channels to volume regulation of cortical astrocytes. Am J Physiol Cell Physiol. 2003;284:C1460–C1467. doi: 10.1152/ajpcell.00603.2002. [DOI] [PubMed] [Google Scholar]
- Parkerson KA, Sontheimer H. Biophysical and pharmacological characterization of hypotonically activated chloride currents in cortical astrocytes. Glia. 2004;46:419–436. doi: 10.1002/glia.10361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston GM, Carroll TP, Guggino WB, Agre P. Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science. 1992;256:385–387. doi: 10.1126/science.256.5055.385. [DOI] [PubMed] [Google Scholar]
- Ransom CB, O’Neal JT, Sontheimer H. Volume-activated chloride currents contribute to the resting conductance and invasive migration of human glioma cells. J Neurosci. 2001;21:7674–7683. doi: 10.1523/JNEUROSCI.21-19-07674.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransom CB, Sontheimer H. BK channels in human glioma cells. J Neurophysiol. 2001;85:790–803. doi: 10.1152/jn.2001.85.2.790. [DOI] [PubMed] [Google Scholar]
- Roberts SK, Yano M, Ueno Y, Pham L, Alpini G, Agre P, LaRusso NF. Cholangiocytes express the aquaporin CHIP and transport water via a channel-mediated mechanism. Proc Natl Acad Sci USA. 1994;91:13009–13013. doi: 10.1073/pnas.91.26.13009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadoun S, Papadopoulos MC, Davies DC, Bell BA, Krishna S. Increased aquaporin 1 water channel expression in human brain tumours. Br J Cancer. 2002a;87:621–623. doi: 10.1038/sj.bjc.6600512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadoun S, Papadopoulos MC, Davies DC, Krishna S, Bell BA. Aquaporin-4 expression is increased in oedematous human brain tumours. J Neurol Neurosurg Psychiatry. 2002b;72:262–265. doi: 10.1136/jnnp.72.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sontheimer H. Ion channels and amino acid transporters support the growth and invasion of primary brain tumors. Mol Neurobiol. 2004;29:61–71. doi: 10.1385/MN:29:1:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi M, Yamashita T, Kumura E, Tamatani M, Kobayashi A, Yokawa T, Maruno M, Kato A, Ohnishi T, Kohmura E, Tohyama M, Yoshimine T. Induction of aquaporin-4 water channel mRNA after focal cerebral ischemia in rat. Brain Res Mol Brain Res. 2000;78:131–137. doi: 10.1016/s0169-328x(00)00084-x. [DOI] [PubMed] [Google Scholar]
- Tietz PS, McNiven MA, Splinter PL, Huang BQ, LaRusso NF. Cytoskeletal and motor proteins facilitate trafficking of AQP1-containing vesicles in cholangiocytes. Biol Cell. 2006;98:43–52. doi: 10.1042/BC20040089. [DOI] [PubMed] [Google Scholar]
- Tsai JC, Goldman CK, Gillespie GY. Vascular endothelial growth factor in human glioma cell lines: Induced secretion by EGF, PDGF-BB, and bFGF. J Neurosurg. 1995;82:864–873. doi: 10.3171/jns.1995.82.5.0864. [DOI] [PubMed] [Google Scholar]
- Uhm JH, Gladson CL, Rao JS. The role of integrins in the malignant phenotype of gliomas. Front Biosci. 1999;4:D188–D199. doi: 10.2741/uhm. [DOI] [PubMed] [Google Scholar]
- Umenishi F, Schrier RW. Identification and characterization of a novel hypertonicity-responsive element in the human aquaporin-1 gene. Biochem Biophys Res Commun. 2002;292:771–775. doi: 10.1006/bbrc.2002.6709. [DOI] [PubMed] [Google Scholar]
- Umenishi F, Schrier RW. Hypertonicity-induced aquaporin-1 (AQP1) expression is mediated by the activation of MAPK pathways and hypertonicity-responsive element in the AQP1 gene. J Biol Chem. 2003;278:15765–15770. doi: 10.1074/jbc.M209980200. [DOI] [PubMed] [Google Scholar]
- Wang W, Hart PS, Piesco NP, Lu X, Gorry MC, Hart TC. Aquaporin expression in developing human teeth and selected orofacial tissues. Calcif Tissue Int. 2003;72:222–227. doi: 10.1007/s00223-002-1014-9. [DOI] [PubMed] [Google Scholar]
- Warth A, Simon P, Capper D, Goeppert B, Tabatabai G, Herzog H, Dietz K, Stubenvoll F, Ajaaj R, Becker R, Weller M, Meyermann R, Wolburg H, Mittelbronn M. Expression pattern of the water channel aquaporin-4 in human gliomas is associated with blood-brain barrier disturbance but not with patient survival. J Neurosci Res. 2007;85:1336–1346. doi: 10.1002/jnr.21224. [DOI] [PubMed] [Google Scholar]
- Weaver AK, Liu X, Sontheimer H. Role for calcium-activated potassium channels (BK) in growth control of human malignant glioma cells. J Neurosci Res. 2004;78:224–234. doi: 10.1002/jnr.20240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidel ML, Ambudkar SV, Smith BL, Agre P. Reconstitution of functional water channels in liposomes containing purified red cell CHIP28 protein. Biochemistry. 1992;31:7436–7440. doi: 10.1021/bi00148a002. [DOI] [PubMed] [Google Scholar]
- Zhang R, Skach W, Hasegawa H, van Hoek AN, Verkman AS. Cloning, functional analysis and cell localization of a kidney proximal tubule water transporter homologous to CHIP28. J Cell Biol. 1993;120:359–369. doi: 10.1083/jcb.120.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]


