Abstract
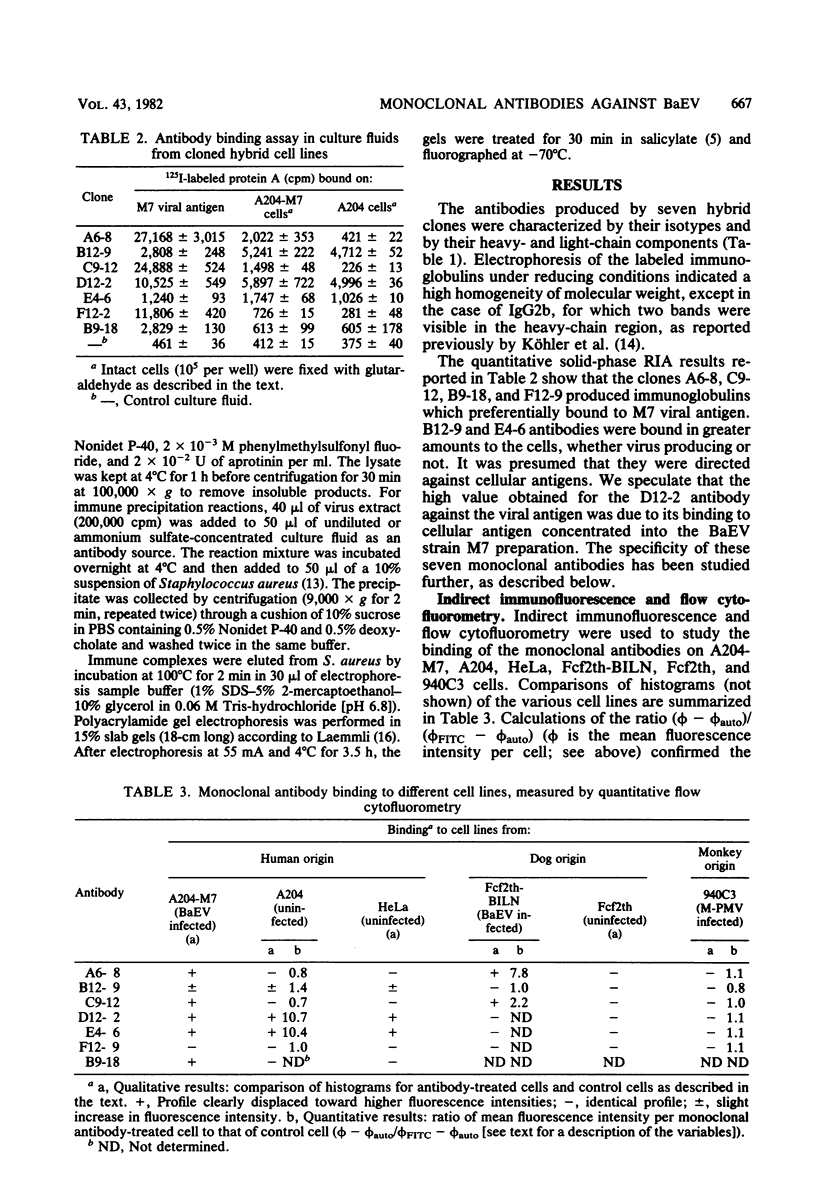
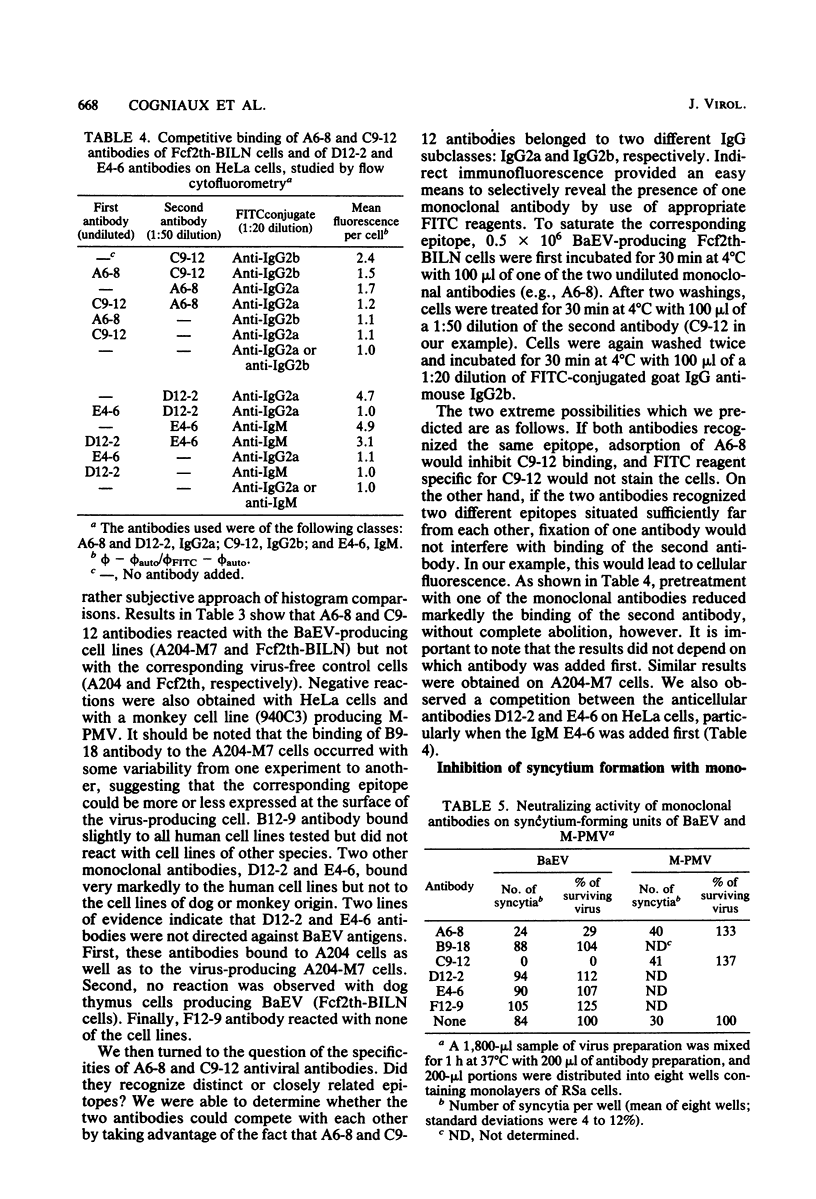
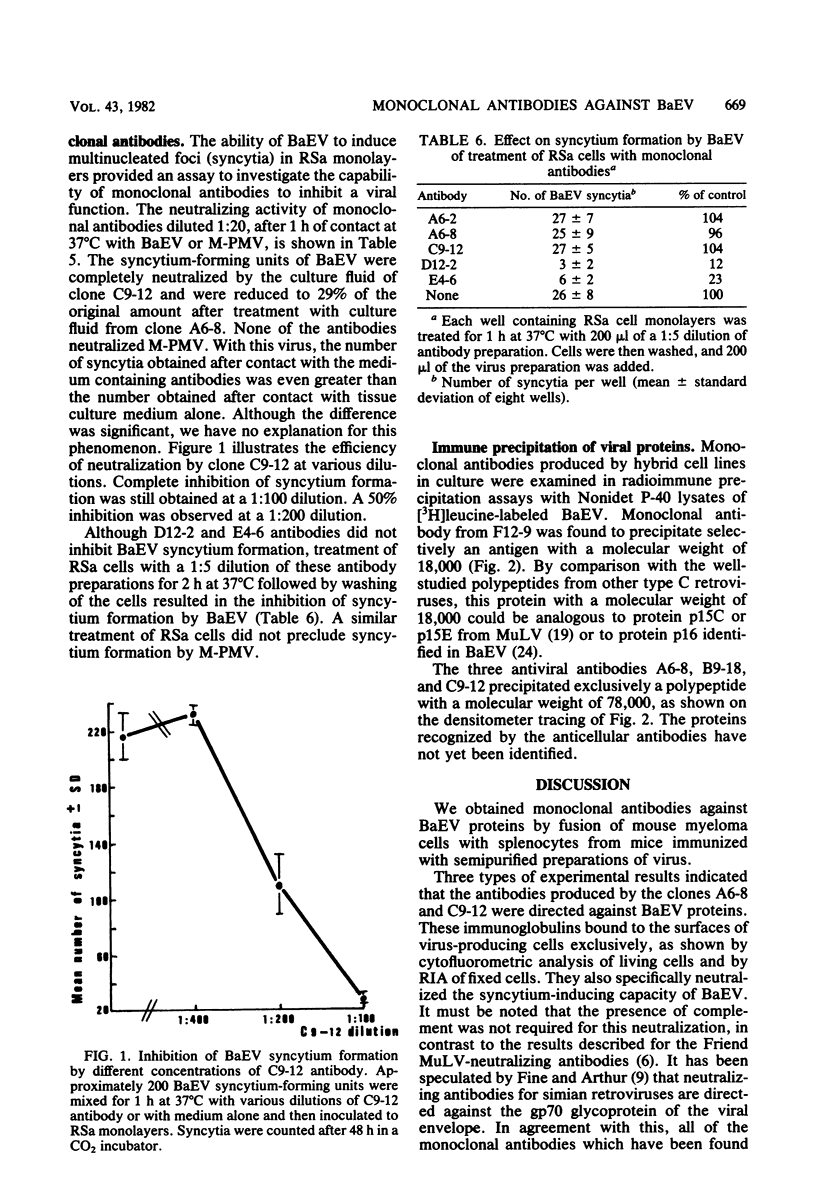
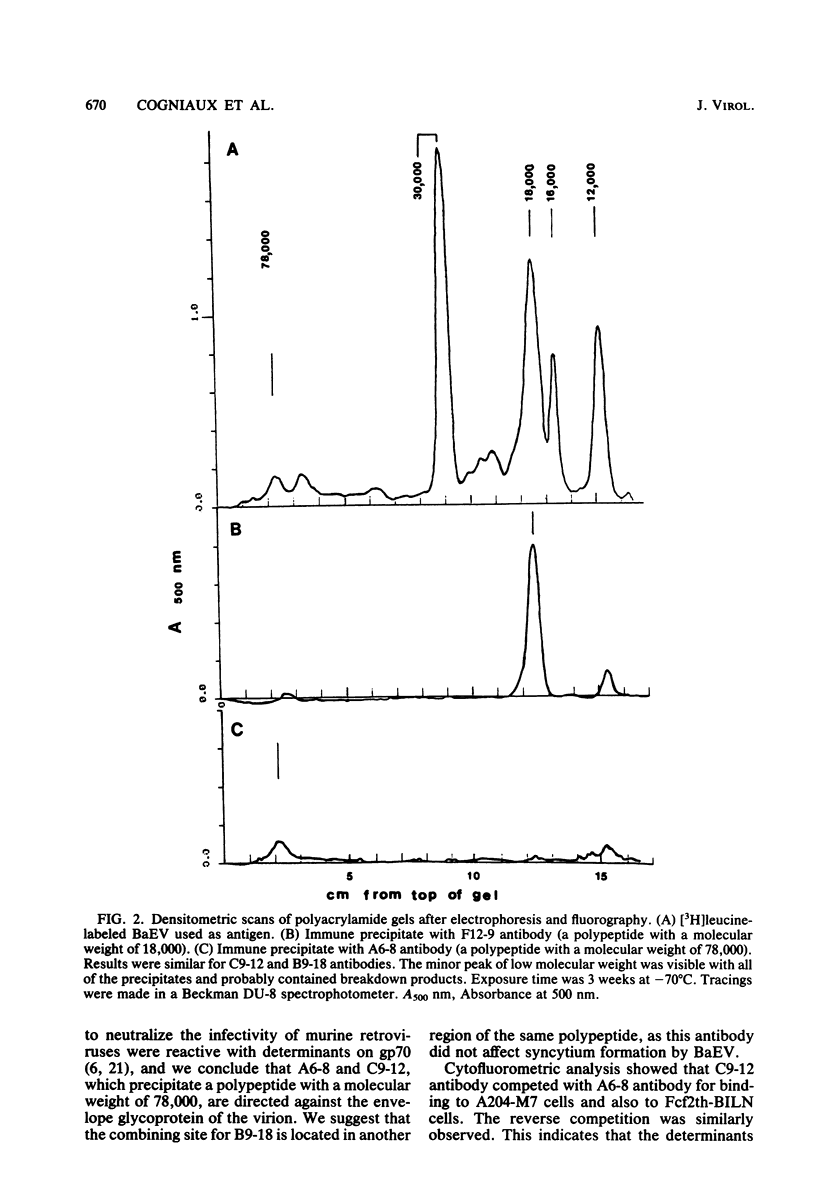
Monoclonal antibodies were produced by murine hybridomas after immunization with semipurified baboon endogenous virus. In a solid-phase radioimmunoassay, two antibodies (F12-9 and B9-18) reacted with viral antigen only. The antibodies A6-8 and C9-12 also reacted with virus-producing cells but not with control cells, whereas antibodies E4-6 and D12-2 bound to virus-free cells as well. The cytofluorometry technique confirmed these results and showed a competition between antibodies A6-8 and C9-12 for binding to virus-producing cells as well as a competition between antibodies D12-2 and E4-6 for binding to virus-free human cells. An immune precipitation assay with disrupted virions indicated that antibodies A6-8, B9-18, and C9-12 were directed against the gp70 glycoprotein, and that antibody F12-9 reacted with a viral antigen with a molecular weight of 18,000. The syncytia induced in RSa cells by baboon molecular weight of 18,000. The syncytia induced in RSa cells by baboon endogenous virus could be inhibited either when antibody A6-8 or C9-12 was combined to the virus or when the RSa cells were treated with the anticellular antibody D12-2 or E4-6. These two effects were not observed with Mason-Pfizer virus. Thus, of three antibodies with specificities for viral gp70, two (A6-8 and C9-12) were directed at viral sites responsible for syncytium formation. Another antiviral antibody (F12-9) reacted with a protein of unknown function with a molecular weight of 18,000. The two anticellular antibodies were directed at similar or neighboring epitopes, which may be situated within the receptor to the virus.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benveniste R. E., Lieber M. M., Livingston D. M., Sherr C. J., Todaro G. J., Kalter S. S. Infectious C-type virus isolated from a baboon placenta. Nature. 1974 Mar 1;248(5443):17–20. doi: 10.1038/248017a0. [DOI] [PubMed] [Google Scholar]
- Benveniste R. E., Todaro G. J. Evolution of type C viral genes: evidence for an Asian origin of man. Nature. 1976 May 13;261(5556):101–108. doi: 10.1038/261101a0. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Chesebro B., Wehrly K., Cloyd M., Britt W., Portis J., Collins J., Nishio J. Characterization of mouse monoclonal antibodies specific for Friend murine leukemia virus-induced erythroleukemia cells: friend-specific and FMR-specific antigens. Virology. 1981 Jul 15;112(1):131–144. doi: 10.1016/0042-6822(81)90619-x. [DOI] [PubMed] [Google Scholar]
- Colcher D., Horan Hand P., Teramoto Y. A., Wunderlich D., Schlom J. Use of monoclonal antibodies to define the diversity of mammary tumor viral gene products in virions and mammary tumors of the genus Mus. Cancer Res. 1981 Apr;41(4):1451–1459. [PubMed] [Google Scholar]
- Fine D. L., Arthur L. O. Expression of natural antibodies against endogenous and horizontally transmitted macaque retroviruses in captive primates. Virology. 1981 Jul 15;112(1):49–61. doi: 10.1016/0042-6822(81)90611-5. [DOI] [PubMed] [Google Scholar]
- Goldberg R. J., Scolnick E. M., Parks W. P., Yakovleva L. A., Lapin B. A. Isolation of a primate type-C virus from a lymphomatous baboon. Int J Cancer. 1974 Dec 15;14(6):722–730. doi: 10.1002/ijc.2910140605. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Kuwata T., Oda T., Sekiya S., Morinaga N. Characteristics of a human cell line successively transformed by Rous sarcoma virus and simian virus 40. J Natl Cancer Inst. 1976 May;56(5):919–926. doi: 10.1093/jnci/56.5.919. [DOI] [PubMed] [Google Scholar]
- Köhler G., Hengartner H., Shulman M. J. Immunoglobulin production by lymphocyte hybridomas. Eur J Immunol. 1978 Feb;8(2):82–88. doi: 10.1002/eji.1830080203. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lostrom M. E., Stone M. R., Tam M., Burnette W. N., Pinter A., Nowinski R. C. Monoclonal antibodies against murine leukemia viruses: identification of six antigenic determinants on the p 15(E) and gp70 envelope proteins. Virology. 1979 Oct 30;98(2):336–350. doi: 10.1016/0042-6822(79)90557-9. [DOI] [PubMed] [Google Scholar]
- Massey R. J., Arthur L. O., Nowinski R. C., Schochetman G. Monoclonal antibodies identify individual determinants on mouse mammary tumor virus glycoprotein gp52 with group, class, or type specificity. J Virol. 1980 Jun;34(3):635–643. doi: 10.1128/jvi.34.3.635-643.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montelaro R. C., Sullivan S. J., Bolognesi D. P. An analysis of type-C retrovirus polypeptides and their associations in the virion. Virology. 1978 Jan;84(1):19–31. doi: 10.1016/0042-6822(78)90215-5. [DOI] [PubMed] [Google Scholar]
- Nowinski R. C., Lostrom M. E., Tam M. R., Stone M. R., Burnette W. N. The isolation of hybrid cell lines producing monoclonal antibodies against the p15(E) protein of ecotropic murine leukemia viruses. Virology. 1979 Feb;93(1):111–126. doi: 10.1016/0042-6822(79)90280-0. [DOI] [PubMed] [Google Scholar]
- Nowinski R. C., Pickering R., O'Donnell P. V., Pinter A., Hammerling U. Selective neutralization of ecotropic murine leukemia virus by monoclonal antibodies: localization of a site on the gp70 protein associated with ecotropism. Virology. 1981 May;111(1):84–92. doi: 10.1016/0042-6822(81)90655-3. [DOI] [PubMed] [Google Scholar]
- Oroszlan S., Nowinski R. C. Lysis of retroviruses with monoclonal antibodies against viral envelope proteins. Virology. 1980 Feb;101(1):296–299. doi: 10.1016/0042-6822(80)90507-3. [DOI] [PubMed] [Google Scholar]
- Scheinberg D. A., Strand M. 55,000-dalton, retrovirus-associated, cell membrane glycoprotein: purification and quantitative measurements of expression in viruses, cells, and tissues. Mol Cell Biol. 1981 Feb;1(2):144–152. doi: 10.1128/mcb.1.2.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen A., Todaro G. J. The genome-associated, specific RNA binding proteins of avian and mammalian type C viruses. Cell. 1977 Jan;10(1):91–99. doi: 10.1016/0092-8674(77)90143-x. [DOI] [PubMed] [Google Scholar]
- Shulman M., Wilde C. D., Köhler G. A better cell line for making hybridomas secreting specific antibodies. Nature. 1978 Nov 16;276(5685):269–270. doi: 10.1038/276269a0. [DOI] [PubMed] [Google Scholar]
- Stocker J. W., Heusser C. H. Methods for binding cells to plastic: application to a solid-phase radioimmunoassay for cell-surface antigens. J Immunol Methods. 1979;26(1):87–95. doi: 10.1016/0022-1759(79)90044-9. [DOI] [PubMed] [Google Scholar]
- Swanstrom R., Shank P. R. X-Ray Intensifying Screens Greatly Enhance the Detection by Autoradiography of the Radioactive Isotopes 32P and 125I. Anal Biochem. 1978 May;86(1):184–192. doi: 10.1016/0003-2697(78)90333-0. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Ogura H., Ocho M., Namba M., Omura S., Oda T. Syncytium formation induced by baboon endogenous virus in several transformed human cell lines. Virology. 1981 Jan 15;108(1):230–234. doi: 10.1016/0042-6822(81)90541-9. [DOI] [PubMed] [Google Scholar]
- de StGroth S. F., Scheidegger D. Production of monoclonal antibodies: strategy and tactics. J Immunol Methods. 1980;35(1-2):1–21. doi: 10.1016/0022-1759(80)90146-5. [DOI] [PubMed] [Google Scholar]


